Jayant Radhakrishnan
Chicago, Illinois, United States
 |
|
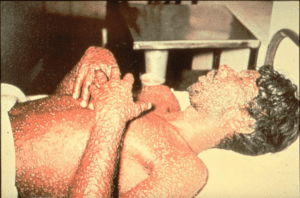
A patient with his whole body covered with smallpox lesions. Source: Centers for Disease Control and Prevention, photo by Barbara Rice. |
Epidemics and pandemics became an issue about 10,000 years ago when hunters and gatherers became farmers and began to live in communities. Smallpox was one of the first lethal infections that spread widely. Its stigmata are seen in Egyptian mummies dating to 1570-1085 BCE. By 1500 CE, in China, India, and parts of Africa, variolation—inoculating with material from a smallpox pustule—was used to immunize susceptible individuals.1 In 1796, Edward Jenner first vaccinated James Phipps with cowpox and after two months exposed him to smallpox. When Phipps remained healthy, Jenner had scientifically proven the commonly held belief that on recovering from cowpox a person is immune to smallpox.2 Since then, vaccines have been created to protect people from many diseases. However, only smallpox has been eradicated from the world, while endemic polio has been eliminated everywhere except in Afghanistan and Pakistan.
Vaccines have evolved considerably over time. First-generation vaccines are made by using the intact but inactivated or attenuated organism as the antigen. Second-generation vaccines manipulate the organism to accentuate a specific component, which is then used as the inciting antigen. Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA) vaccines constitute the third generation of vaccines.
First-generation vaccines
Inactivated vaccines are made by isolating the pathogen, growing it in an animal model or in tissue culture, and then exposing it to a noxious agent such as heat or chemicals to render it noninfectious without affecting its immunogenicity. The advantages of these vaccines are that they cannot revert back into the original pathogen and are not likely to interfere with each other if used in combination. Furthermore, the methodology is well established, they are relatively easy to manufacture, and they can be shipped and stored for long periods of time at 2-8°C. Finally, they may be used safely in immunocompromised individuals.3 The major disadvantage is that two or three initial injections and subsequent booster doses are required to mount an adequate immune response. Jonas Salk’s inactivated polio vaccine (IPV) was made by this technique.4 Hepatitis A, rabies, and an injectable influenza vaccine are also made from killed organisms.
Sinovac and Sinopharm vaccines from China and Covaxin from India are inactivated vaccines against COVID-19 and at least two more inactivated vaccines are currently being evaluated against SARS-CoV-2.5
Live attenuated vaccines are created by isolating the causative organism and serially passaging it (growing it sequentially) in a foreign host to weaken its pathogenicity to such a degree as to render it harmless before injecting it into the subject.6 Though no longer pathogenic, the organism maintains its antigenicity and produces an immediate, strong, and long-lasting immune response in everyone except the immunocompromised. This vaccine can also be produced rapidly. However, it can create serious problems in the immune-compromised and there is some concern that it could revert back into its previous live, pathogenic form. These vaccines must be kept under strict cold conditions from manufacture to administration. The first such vaccine was Bacillus Calmette Guerin (BCG) for tuberculosis. The vaccines for measles, mumps, rubella, chickenpox, yellow fever, and also the intranasal influenza (FluMist) and Sabin oral polio vaccines are in this category.
Viruses have also been attenuated by reverse genetics. Once the genetic sequence that controls a particular phenotype is identified, that gene can be silenced, subjected to targeted disruption, or to insertional or chemical mutagenesis creating a virus with the desired phenotype.7 The yearly influenza vaccines are made in this manner.
Second-generation vaccines
Subunit vaccines are developed by using only the antigenic part of the organism.8 This may be achieved by growing the organism and then separating off the desired antigen or by using DNA technology to create antigen molecules. The molecule could be a protein, polysaccharide, or peptide. These vaccines are safe since the organism has been killed, however, they are less immunogenic than live attenuated vaccines, and it may be difficult to isolate the antigen to evoke a specific immune response. The Hepatitis B vaccine belongs in this category. Similar vaccines are also being evaluated against cancer and the Human Immunodeficiency Virus (HIV).
Novavax has developed a protein subunit vaccine against SARS-CoV-2.
Conjugate vaccines also use only a portion of the organism. Combining a weak antigen with a strong carrier antigen stimulates a powerful immune response to the weak antigen. For example, if an organism has a polysaccharide coating, the immature immune system of infants does not mount an immune response as it does not recognize the antigen. However, if the polysaccharide is attached to a stronger protein, the same immature immune system mounts a vigorous response to the protein and to the associated polysaccharide. Some examples are the pneumococcal conjugate (Prevnar) and the Hemophilus Influenza conjugate (HiB) vaccines.
The Johnson and Johnson, AstraZeneca, and Russian Sputnik V vaccines against COVID-19 are based on this technique.
Toxoid vaccines are employed where the toxin secreted by the organism causes the affliction, not the organism itself. The toxin is inactivated by formalin into a harmless toxoid. Toxoids are used for diphtheria and tetanus.
Recombinant vector vaccines are composed of attenuated organisms that carry DNA to cells and stimulate an immune response. A segment of the viral gene is inserted into the gene of a yeast cell. When this modified yeast cell grows, it produces a pure antigen. To date, vaccines against HIV, Human Papilloma Virus (HPV), hepatitis B, rabies, and measles have been developed in this manner.9
The Chinese CanSinoBio vaccine against COVID-19 is a recombinant adenovirus vaccine.
Third-generation vaccines
DNA vaccines are made by introducing DNA encoded for a specific antigen into the body. The cells take this up and as part of their normal metabolism synthesize proteins based upon the genetic code of the plasmid they assimilated. These vaccines are also made by enveloping the DNA in a protein to enhance its ability to enter the cell. Such vaccines could be as potent as live vaccines without risk of precipitating the disease since they would not contain the disease producing part of the organism. In addition, they would stimulate the B and T cells, be stable, and should be relatively easy to manufacture in large amounts. On the other hand, concerns have been raised regarding integration into the host’s chromosomes and germline alterations, inappropriate immune responses that could express cytokines or co-stimulatory molecules, development of antibodies against the DNA injected resulting in undesired autoimmune reactions, and biological activity of the expressed antigen or expression of other risk-producing gene sequences in mammalian or bacterial cells. Also, while immune responses in animal models have been encouraging, it is not so in humans and various maneuvers are being tested to enhance the human immune response. Vaccines for Zika, herpes, and influenza are being developed by this technique. No DNA vaccine has been approved for human use in the USA to date.10,11
Messenger RNA vaccines are the latest type of vaccine. mRNA carries instructions from DNA to ribosomes in the cytoplasm of cells to produce a specific protein and to display it on the surface so as to trigger an immune response. They were developed against COVID-19 starting in February 2020 and received Emergency Use Authorization (EUA) from the US Food and Drug Administration (FDA) by December. This seems extremely hasty, but it is the culmination of many years of work. Scientists had been trying to use mRNA for over thirty years but they could not get over the following hurdles: mRNA was unstable, highly immunogenic, and the in vivo delivery system was inefficient. After years of work, in 2005 Katalin Karikó and Drew Weissman identified that a nucleoside was alerting the body’s immune system to react. They overcame the problem by incorporating modified nucleosides in the mRNA.12 These vaccines have many advantages. Since mRNA is non-infectious and non-integrating (it does not affect the DNA), there is no risk of infection or genetic mutation. It is degraded by normal cellular processes and its half-life can be modified. Its immunogenicity can also be down-regulated. mRNA can be rendered more stable and it is highly translatable. Rapid uptake and expression of mRNA in cytoplasm can be achieved by formulating it into carrier molecules. mRNA vaccines can be administered repeatedly as they do not generate anti-vector immunity. Finally, these vaccines can be manufactured rapidly and inexpensively.13
Moderna and Pfizer have used this technique to make their vaccines for COVID-19.
Another benefit of this methodology is that once a platform has been developed, vaccines could be produced for infectious diseases, cancers, or autoimmune diseases by inserting the appropriate mRNA sequence. It could also be used to redress protein deficiencies in organs or in diseases such as cystic fibrosis.
Some future possibilities. DNA synthesis and sequencing can be used to create viruses on which to study the effects of vaccines14 and vaccines can be developed by encoding the antigen protein of a disease in the genome of plant tissues.15
Jenner personally distributed his vaccine to anyone who asked for it, Salk purposely did not patent his discovery, and not only did Sabin not patent his vaccine, he donated his collection of polio virus strains to the World Health Organization (WHO) in 1972. All three pioneers wanted to ensure that their vaccine would be available when and where needed. The situation now is very different because a handful of countries and pharmaceutical companies are in control. Hence, after the Ebola epidemic of 2014-2016 the WHO allotted considerable resources to form a coalition that would cooperate to rapidly identify and equitably manage future epidemics of diseases known at the time, and any caused by a hypothetical future Disease X.16,17 Unfortunately, when “Disease X” showed up in the form of the SARS-CoV-2 pandemic, the plan fell apart, exposing gross inequities around the world because resources have not been pooled and shared as the WHO had planned.18 As the Kikuyu of Kenya say, “When elephants fight it is the grass that suffers.”
Bibliography
- Tishma M (2020): The global journey of variolation. Hektoen International Sections/Infectious Diseases/Summer 2020.
- Riedel S (2005): Edward Jenner and the history of small pox and vaccination. Proc Bayl Univ Med Cent. 18(1):21–25 doi: 10.1080/08998280.2005.11928028.
- Sangarani S (2018): Recent development of inactivated vaccine-a review. Internat J Sci Res 7(11):22-23 doi: 10.21275/ART20192407
- Baicus A (2012): History of polio vaccination. World J Virol 1(4):108-114 doi:10.5501/wjv.v1.i4.108.
- Al Kaabi N, Zhang Y, Xia S, Yang Y, Al Qahtani MM, Abdulrazzaq N, et al (2021): Effect of 2 inactivated SARS-CoV-2 vaccines on symptomatic COVID-19 infections in adults: A randomized clinical trial. JAMA published online May 26, 2021 doi:10.1001/jama.2021.8565.
- Badgett MR, Auer A, Carmichael LE, Parrish CR, Bull JJ (2002): Evolutionary dynamics of viral attenuation. J Virol 76(20):10524-10529 doi:10.1128/JVI.76.20.10524-10529.2002.
- Tierney MB, Lamour KH (2005): An introduction to reverse genetic tools for investigating gene function. APSnet. The American Phytopathological Society. The Plant Health Instructor. doi: 10.1094/PHI-A-2005-1025-01.
- Vartak A, Suchek SJ (2016): Recent advances in subunit vaccine carriers. Vaccines (Basel) 4(2):12 doi: 10.3390/vaccines4020012. Published online April 19, 2016
- Bull JJ, Nuismer SL, Antia R (2019): Recombinant vector vaccine evolution. PLoS Comput Biol 15(7): e1006857 https://doi.org/10.1371/journal.pcbi.1006857.
- World Health Organization (2007): Guidelines for assuring the quality and nonclinical safety evaluation of DNA vaccines WHO technical report series No 941Annex 1.
- Sheets R, Kang H-N, Meyer H, Knezevic I (2020): Meeting report WHO informal consultation on the guidelines for evaluation of the quality, safety, and efficacy of DNA vaccines. Geneva, Switzerland, December 2019. npj/Vaccines 5(52) Published online 18 June 2020. www.nature.com/npj/vaccines https://doi.org/10.1038/s41541-020-0197-2.
- Karikó K, Buckstein M, Houping N, Weissman D (2005): Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity 23(2):165-175 doi: 10.1016/j.immuni.2005.06.008.
- Pardi N, Hogan MJ, Porter FW, Weissman D (2018): mRNA vaccines-a new era in vaccinology. Nature reviews 17:261-279 published online 12 Jan 2018. doi:10.1038/nrd.2017.243.
- Wimmer E, Mueller S, Tumpey TM, Taubenberger JK (2009): Synthetic viruses: A new opportunity to understand and prevent viral disease. Nat Biotechnol 27(12):1163-1172. Published online Dec 9 2009. doi: 10.1038/nbt.1593.
- Laere E, Ling APK, Wong YP, Koh RY, Lila MAM, Hussein S (2016): Plant-based vaccines: Production and challenges. J Botany Article ID 4928637, 11 pages http://dx.doi.org/10.1155/2016/4928637.
- WHO: An R&D blueprint for action to prevent epidemics: Accelerating R&D and saving lives. Update 2017. https://www.who.int/blueprint/about/en/
- WHO: R&D blueprint list of priority diseases. Update 2017. https://www.who.int/blueprint/priority-diseases/en/
- Baumgaertner E (2021): Vaccine companies and the US government snubbed the WHO initiative to scale up global manufacturing. Los Angeles Times April 30, 2021.
JAYANT RADHAKRISHNAN, MB, BS, MS (Surg), FACS, FAAP, completed a Pediatric Urology Fellowship at the Massachusetts General Hospital, Boston following a Surgery Residency and Fellowship in Pediatric Surgery at the Cook County Hospital. He returned to the County Hospital and worked as an attending pediatric surgeon and served as the Chief of Pediatric Urology. Later he worked at the University of Illinois, Chicago from where he retired as Professor of Surgery & Urology, and the Chief of Pediatric Surgery & Pediatric Urology. He has been an Emeritus Professor of Surgery and Urology at the University of Illinois since 2000.

Leave a Reply