S.E.S. Medina
Benbrook, Texas, United States
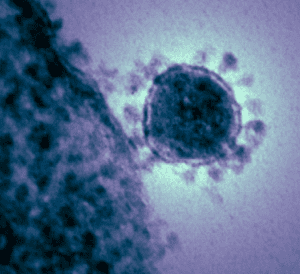 |
| Coronavirus: Protein Spike Corona. A colorized transmission electron micrograph of the Middle East respiratory syndrome-related Coronavirus (MERS-CoV) that emerged in 2012. November 19, 2012. Public domain image from National Institutes of Health. Source. |
A tiny mote of moisture, buoyed by silk-soft wind currents, is kicked and coaxed along a random path in space. The droplet carries a microscopic stowaway; a translucent, spherical, protein-encased, fatty bubble filled with the ostensibly lifeless essence of COVID-19, the virus known as SARS-CoV-2. Anonymous among the swirling detritus of the atmosphere, the tumbling Trojan globule is inhaled by a new host trillions of times its size. The droplet containing the minuscule invader careens haphazardly inside the unsuspecting victim’s cavernous respiratory tract until slamming against a humongous cell.
This diminutive entity, outlined by sparks of whirling electrons coruscating up and down endless chains of atoms, is studded with minuscule, spike-like proteinaceous protuberances and resembles a miniature medieval battle mace. The spike’s barbaric appearance belies an elegant operation, for it is the magical “skeleton key” that gains entry into the domicile of its victim. Like the guiding hand of a danseur noble leading his danseuse, one of the seventy-odd surface spikes interlocks with its complement—the Angiotensin-Converting Enzyme-2 receptor (ACE-2) (1)—on the outer periphery of the host’s respiratory cell. This facilitates invasion by splitting the SARS-CoV-2’s spike protein into its active, binary components via a membrane-bound, host serine protease—TMPRSS2.1 Half of the spike—its S1 subunit—allows firm attachment of the virus to the surface of the target cell, anchoring the invader to its victim. The S2 subunit ensures entry by catalyzing fusion between the fatty membrane of the virus with that of the gargantuan cell.
For over 55 million years, SARS-type coronaviruses have co-existed with flying nocturnal mammals,2 with its most recent, common genetic ancestor born as of 8000 BCE.3
The current worldwide pandemic of SARS-CoV-2 reputably started within the city of Wuhan, in the central Chinese province of Hubei,4 home to a large, open “wet” market where live animals (including bats) are sold for food, and coincidentally, a level-4 biosafety hazard lab (BSL-4), the Wuhan Institute of Virology, where extensive studies of coronaviruses are conducted.5
With full access to the host’s cytoplasm, the SARS-CoV-2’s single-stranded RNA genome hijacks the cell’s manufacturing abilities by binding to protein-synthesizing ribosomes anchored to the perinuclear, rough endoplasmic reticulum. Ribosomal translation of the encrypted viral RNA results in the synthesis of several polyproteins, which auto-catalyze and cleave into smaller, functional fragments, the raw materials necessary for replication of the invader’s RNA genome, and various key structural and non-structural proteins required for the delicate birthing of new siblings.
Further augmenting infectivity, the SARS-CoV-2 coerces the invaded, zombie-like cell to grow and radially array actin-rich, cytoskeletal branched filopodia (tentacles).6 Each of the filopods contains multiple M-protein clusters capable of producing more SARS-CoV-2. The sword-like appendages forcibly pierce the membranes of adjacent, innocent host cells, reaching deep into their cytosols. These protuberances burst and release fresh kin, which commandeer the newly violated victim.
The host’s immune system offers little resistance because of viral suppression of Interleukin-1 and Interleukin-3, chemokines necessary for the activation of antiviral defenses,7, 8 silent virion shedding, and subsequent infestation of new hosts. This immunological passivity permits widespread, asymptomatic infection throughout the victim’s respiratory tract, extending into the bloodstream, and allowing an invasion of heart and neuronal cells, which have an abundance of ACE-2 receptors.9
With infiltration of these delicate tissues, the adroit clinical complications of the disease become manifest, triggering cardiac arrhythmias, encephalopathies, and stroke via involuntary activation of the host’s blood coagulation.10-13 This subversive activity takes its toll, causing widespread multi-organ damage and the belated awakening of the victim’s immunological system. The ACE-2 employed by SARS-CoV-2 as its entry receptor is expressed on lung type II alveolar cells, arterial and venous endothelial cells, enterocytes, and arterial smooth muscle cells.
With the unfettered circulation of infectious viral particles and components, lymphocytes routinely patrolling the body eventually recognize the unwelcome, invading alien through complicated, eons-old physical and chemical interactions between the virion and the T-cell’s surface receptors. Activated T-cells and macrophages hurriedly synthesize and secrete a multitude of potent substances called cytokines, such as Interleukin-6, Interleukin-8, and Tumor Necrosis Factor-α,14,15 which precipitate fever, tissue edema, joint pain, fatigue, the enlistment of hepatocytes in the production of key acute phase inflammatory proteins, and the activation and recruitment of antibody-producing B-cells.16
This is often the end of the story for SARS-CoV-2 and its exploited victim, with the immune system rallying, making antibodies, and thus destroying SARS-CoV-2’s capacity to survive and reproduce. But horrifically, in a tiny fraction of infected humans, the body’s immunological reaction becomes deranged, precipitating a “cytokine storm”: severe, out-of-control cellular damage accompanied by reduced lymphocyte counts affecting CD3+, CD4+, and CD8+ T-cells.17 This auto-inflammatory response produces far more spoliation to internal organs beyond that perpetrated by the nefarious activities of SARS-CoV-2.17
However, it has been determined that SARS-CoV-2 also triggers a potent autoimmune reaction in a miniscule fraction of infected individuals, with the synthesis of autoantibodies directed against various targets, among them phospholipids and type-I interferons. A powerful link between relentless infection and an intense extra-follicular, B-cell proliferation with serological features evocative of SLE pathogenic auto-reactivity has been detected. A positive ANA of at least ≥ 1:80 with a speckled pattern occurred in a sizeable number of affected patients, the entry threshold value for SLE classification. Eighty-one percent display titers of ≥1:160, a value obtained in <5% of the general population, with upper limits of 1:320-1:640.18 Two of twenty-two ANA+ individuals had detectable Anti-RNP and anti-centromere IgG titers. However, anti-dsDNA was not identified. The frequent appearance of antibodies such as rheumatoid factor (10/52), or autoantibodies against phospholipids (3/52), prothrombin (2/52), and c-ANCA (2/52), with or without ANA reactivity, shows broad autoimmune targeting. This discovery implies uncomplicated laboratory testing for ANA or RF could be employed to identify these subjects. Clinical correlation with these positive autoantibody tests suggests they may be partially responsible for the generation of dangerous blood clots forming systemically in ICU patients.18
Therefore, treatment with dexamethasone, which is used to control “flare-ups” of autoimmune disorders, could be effective in managing individuals with the most aggressive disease. Unfortunately, these auto-inflammatory/autoimmune responses are not short-lived, often outlast the acute infection, and possibly contributing to the ongoing symptoms suffered by a growing number of “long-hauler” COVID-19 patients.18
Hematological investigation suggests the Alternative Complement Pathway (APC) is activated, causing the disseminated intravascular coagulation (DIC) observed in SARS-CoV-2 infection. There is evidence the SARS-CoV-2 spike protein (subunit 1 and 2), and not the N protein, is what activates the APC. An interaction between the viral spike proteins and the target cell’s non-activator surfaces renders them activator surfaces by specifically preventing the deactivation of APC Convertase on the cell surface. Spike proteins attach to the target cell by binding to surface glycosaminoglycans (heparan sulfate and α2,3 and α2,6 sialylated N-glycans), permitting the interaction with entry receptors and facilitating membrane fusion with host cells.19
Structural studies of the Receptor Binding Domain in SARS-CoV-2 spike protein S1 subunit demonstrate a minute, positively-charged region capable of bonding to negatively-charged heparan sulfate. Activation of APC Convertase likely accounts for many of the clinical manifestations (thrombocytopenia, microangiopathy, thrombophilia, and renal injury) of SARS-CoV-2 that are seen in other disorders such as atypical Hemolytic Uremic Syndrome (aHUS) and Catastrophic Anti-Phospholipid Antibody Syndrome (CAPS), both of which are complement-mediated. The complement activation induced by SARS-CoV-2 spike proteins blocks Factor D Inhibitor (ACH-145951). Inhibition of C5 stops the accumulation of C5b-9 on cells, but not C3c. Factor D inhibition interrupts both C3c and C5b-9 accretion. As Factor D is unique to the APC, this information suggests that S1 and S2 subunits of SARS-CoV-2 spike protein activate complement principally through the APC.19 The refractory thrombotic microangiopathy, diffuse endothelial damage, hypercoagulability, and the inflammatory environment connected to cataclysmic COVID-19 infection has led to the belief that extreme complement activation is responsible for the end-organ deterioration.
In humans struggling with COVID-19 pneumonia, C5b-9, C4d, and Mannan-binding lectin Serine Protease (MASP) 2 are detectable in the lung’s microvasculature. Skin lesions associated with COVID-19 infection demonstrate SARS-CoV-2 spike proteins co-localizing with C4d and C5b-9 in the cutaneous microvasculature. One hundred-fifty patients with COVID-19 acute adult respiratory distress syndrome (ARDS) investigated in a prospective cohort study discovered a high incidence of pulmonary emboli (17%) despite aggressive prophylactic anticoagulation. Thrombosis occurring in COVID-19 is incompletely responsive to anticoagulation (heparin treatment resistance and clotting developing in spite of appropriate prophylactic anticoagulation), and is characteristic of complementopathies such as cold-agglutinin disease and paroxysmal nocturnal hemoglobinuria.19
In the acute phase of virus infection, several complement proteins including complement 6 (C6), complement factor B (CFB), Properdin (CFP), and Carboxypeptidase-N catalytic chain (CPN1) are detectable.19 It will be vital to establish whether patients with extreme forms of COVID-19 possess variants in complement regulatory genes. In contrast to older human victims, very young hosts are often asymptomatic when infected by SARS-CoV-2. However, a tiny fraction of young people also suffer from a cytokine storm, experiencing severe clinical symptoms such as respiratory failure, shock, stroke, and even death.20
Unraveling the mysteries surrounding the protean immunological manifestations of SARS-CoV-2 will hopefully permit improved management and treatment of seriously afflicted patients.
Bibliography
- Hoffmann et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020; https://doi.org/10.1016/j.cell.2020.02.052
- Fenner’s Veterinary Virology (Fifth Ed.). Academic Press. 2017. 435–461. doi:10.1016/B978-0-12-800946-8.00024-6 (https://doi.org/10.1016%2FB978-0-12-800946-8.00024-6). ISBN 978-0-12-800946-8.
- Hou Y, et al. Angiotensin-Converting Enzyme 2 (ACE2) Proteins of Different Bat Species Confer Variable Susceptibility to SARS-CoV Entry – Arch Virol. 2010; 155:1563-1569.
- Kinetz, E. Where Did They Go? Millions Left City Before Quarantine. AP News. February 9, 2020. https://apnews.com/article/c42eabe1b1e1ba9fcb2ce201cd3abb72
- Frayer JM, Chow D. Inside the Chinese Lab Central to the Search for the Coronavirus’ Origin. NBC News. August 10, 2020. https://www.nbcnews.com/news/world/inside-wuhan-lab-center-coronavirus-storm-n1236254
- Bouhaddou M, et al. The Global Phosphorylation Landscape of SARS-CoV-2 Infection. Cell. 2020; 182: 1-28
- He, X., et al. Temporal Dynamics in Viral Shedding and Transmissibility of COVID-19. Nat Med. 2020; https://doi.org/10.1038/s41591-020-0869-5
- Blanco-Melo D, et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell. 2020; DOI: 10.1016/j.cell.2020.04.026
- Zheng YY, et al. COVID-19 and the Cardiovascular System. Nat Rev Cardiol. 2020 Mar 5; doi: 10.1038/s41569-020-0360-5.
- Kochi AN, et. al. Cardiac and Arrhythmic Complications in Patients with COVID-19. J Cardiovasc Electrophysiol. 2020; 31:1003-1008
- Mao L, et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. Published online April 10, 2020; doi:10.1001/jamaneurol.2020.1127
- Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of Ischaemic Stroke Associated with COVID-19. Journal of Neurology, Neurosurgery & Psychiatry. Published Online First: 30 April 2020; doi: 10.1136/jnnp-2020-323586
- Giannis D, et al. Coagulation Disorders in Coronavirus Infected Patients: COVID-19, SARS-CoV-1, MERS-CoV and Lessons from the Past. J Clin Virol. 2020; 127: 1-4.
- Dinarello CA – Interleukin-1 in the Pathogenesis and Treatment of Inflammatory Diseases. Blood. 2011; 117(14):3720-3732.
- Tanaka T, et al. IL-6 in Inflammation, Immunity and Disease. Cold Spring Harb Perspect Biol. 2014; 6:1-16.
- Prete M, et al. SARS-CoV-2 Inflammatory Syndrome. Clinical Features and Rationale for Immunological Treatment Laboratory Findings. Int J Mol Sci. 2020; 21: 3377-3390.
- Zhang X, et al. Viral and Host Factors Related to the Clinical Outcome of COVID-19 – Nature. 2020; DOI: 10.1038/s41586-020-2355-0
- Woodruff MC, et al. Clinically Identifiable Autoreactivity is Common in Severe SARS-CoV-2 Infection. MedRxiv preprint Oct 2020 https://doi.org/10.1101/2020.10.21.20216192
- Yu J, et al. Direct Activation of the Alternative Complement Pathway by SARS-CoV-2 Spike Proteins is Blocked by Factor D Inhibition. Blood. Oct 29, 2020. 29; 136(18):2080-2089. doi: 10.1182/blood.2020008248.
- Riphagen S, et al. Hyperinflammatory Shock in Children during COVID-19 Pandemic. Lancet – Published Online May 6, 2020 https://doi.org/10.1016/ S0140-6736(20)31094-1
S. E. S. MEDINA, MD, is a retired Internal Medicine specialist with a sub-specialty in Infectious Diseases. His initial medical training took place at the New York University School of Medicine in the late 1970s when the AIDS epidemic was just beginning, working with severely ill HIV infected patients. Clinical research under Dr. Linda Laubenstein during his fourth year at the NYU Medical School resulted in a contributing paper which was included in the first medical textbook on AIDS.
Dr. Medina would like to acknowledge the creative and editorial contributions of his nephew and godson, David I. Banchs, in the writing of this story.

Leave a Reply