Putzer J. Hung
Saint Louis, Missouri, United States
“What you do in this world is a matter of no consequence. The question is, what can you make people believe that you have done?”
– Sherlock Holmes, A Study in Scarlet
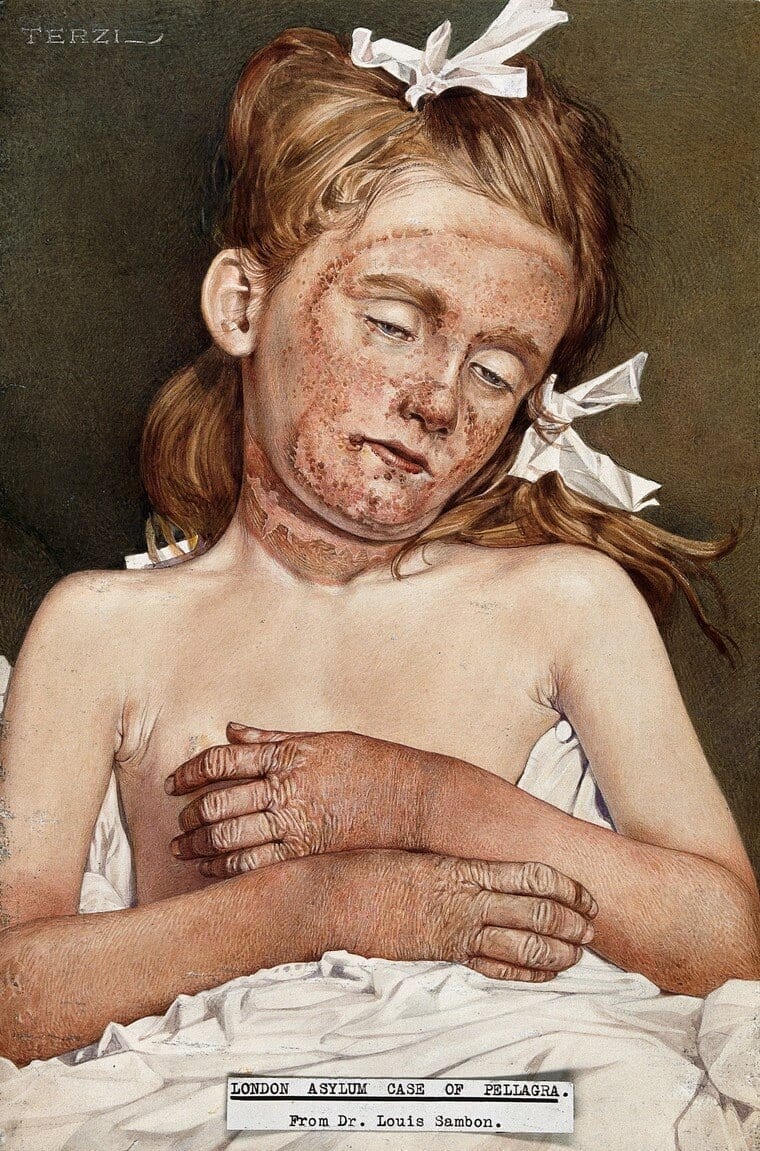
Beginning in 1902, a strange epidemic struck the southern United States. Victims, often women and children of impoverished means, presented with red, scaly rashes that were exacerbated by sunlight. As the disease progressed, they suffered increasingly from weight loss, diarrhea, depression, and even “madness,” speaking incoherently to figments of their own imaginations. Come winter, these symptoms would inexplicably recede, only to flare up again in the following spring, and death usually resulted after a protracted illness. Incidences of “spring sickness” (as it was colloquially called) rose rapidly: by 1912, nearly 16,000 cases had been reported across eight Southern states, with a mortality rate reaching 40%.1 Although new to American physicians at the time, this malady—now known as pellagra (stemming from the Italian phrase pelle agra for “rough skin”)—had been endemic in Europe since the 1730s. The diagnosis was finally made in the summer of 1908 by Dr. James Babcock who, on a visit of several infirmaries for pellagrins in Italy, recognized the same tell-tale clinical signs he had seen among his patients at the South Carolina State Hospital for the Insane.2 On returning to the U.S., Babcock organized the first English-language conference on the disease, thus propelling pellagra into the national spotlight and sparking a bitter scientific debate that would span the next three decades.
Although both European and American medical establishments had discerned a long-standing association between “Indian corn” (maize) and pellagra, professional opinion diverged sharply over the cause of the disease. The germ theory model, proposed by the flamboyant Italian-English physician Dr. Louis Sambon, alleged that pellagra was a vector-borne zoonosis like malaria and leishmaniosis. Sambon had based his reasoning on the preferential distribution of pellagra in northern Italy among rural populations living by rivers and streams. Corn was not the culprit per se, he remarked, but “it is in the maize field that the peasant comes in touch with the specific agent of pellagra, and possibly through the agency of some biting fly.”3 The countervailing model, put forth by the Polish biochemist Casimir Funk in 1912, postulated that pellagra was caused by the deficiency of a specific nutrient (“vital amine”) in corn. Funk had observed striking similarities between pellagra, beriberi, rickets, and scurvy. All these diseases, he noted, “break out in countries where a certain unvarying diet is partaken for long periods.”4 Since beriberi and scurvy “can be prevented and cured by the addition of certain preventative substances [in food],” Funk hypothesized that pellagra could be too.4
In response to public anxiety over the “national calamity,” the U.S. Public Health Service (PHS) appointed in 1909 Dr. Claude Lavinder, a highly reputed epidemiologist, to track down the source of the outbreak. Pursuing Sambon’s line of thought, Lavinder attempted unsuccessfully for the next five years to demonstrate the infectious transmission of pellagra by injecting rabbits, guinea pigs, chickens, and monkeys with body fluids collected from deceased pellagrins.5 Concurrently, in 1912, the privately sponsored Thompson-McFadden Commission launched an independent epidemiological study of pellagra in South Carolina. While a negative correlation between dairy consumption and pellagra was found, it was dismissed because milk “did not fully insure against the development of the disease.”6 The Commission ruled out a nutritional cause, citing that pellagra was rarely seen in nursing infants (unlike beriberi) and was actually more prevalent among whites than blacks, who were perceived to have a less healthy diet; instead, it suggested a pathogenic etiology, but as with Lavinder, was unable to identify the responsible agent.7, 8
In 1914, Lavinder sought relief from his work and was replaced by Dr. Joseph Goldberger, who, as a Hungarian-Jewish doctor from New York might have initially seemed like an odd fit for the job. Yet he was energetic, meticulous, and importantly, possessed extensive experience in dealing with contagious diseases such as malaria, dengue, and typhoid fever. Traveling south, Goldberger visited textile towns, orphanages, and mental asylums—places where pellagra ran rampant—and was appalled by the level of poverty he witnessed. Southern economy and farmlands had become dominated by “King Cotton,” forcing the poor to subside on a cheap, monotonous diet of three M’s: meal (corn flour), meat (fatback), and molasses.9 Goldberger quickly noticed that pellagra only affected these people and never the affluent landowners or doctors. Thus, after also failing to transfer the disease from humans to monkeys, he began to suspect that malnourishment lay at the root of the epidemic. In a landmark two-year study conducted at two orphanages in Mississippi and a psychiatric hospital in Georgia involving over 700 subjects, Goldberger and his colleagues showed that by simply changing the usual diet to one “enjoyed by the well-to-do” consisting of milk, eggs, beans, and peas, they could almost entirely eradicate the disease: after a year, there was only a single recurrence among 400 pellagrins and no incident cases.10 More stunning were the results of Goldberger’s subsequent Rankin Prison Farm experiment, in which he was able to induce pellagra in a group of healthy convicts (who had volunteered in exchange for pardons) by feeding them grits, biscuits, and syrup for six months.11
When Goldberger presented his findings in 1915, advocating that the solution was to “improve economic conditions” and to “make other class of foods cheap and readily accessible,” he was met by a wave of disbelief and derision.11 His most vocal opponents included not only members of the Thompson-McFadden Committee, which felt offended by his criticisms of its methodologies, but also Southern public officials and business leaders, who considered his conclusion that pellagra was a consequence of regional famine as an attack on their way of life. Although furious at the prejudice he faced, Goldberger continued his work. In the spring of 1916, as a defiant display of proof, he and his close associates hosted a series of “filth parties,” in which they tried to acquire pellagra by eating and inoculating themselves with the skin, blood, urine, feces, or nasal discharges of pellagrins. “If anyone ever got pellagra that way, we three should certainly have it good and hard!” he declared—yet no one contracted the disease.12, 13 Moreover, along with the PHS, Goldberger followed up on the Thompson-McFadden report by surveying an overlapping set of seven cotton-mill villages in South Carolina and quantifying food supplies at the individual level (which the Commission had failed to do).8, 14 The only significant difference he found between the villages with the highest and lowest incidences of pellagra was the availability of fresh produce, the latter having access to well-stocked stores and nearby farms.8, 14 Despite the broad media coverage received by the study, Southern pride maintained its denial of the harsh truth. Many of the institutions where Goldberger had eliminated the disease resumed their former meal plans, and Southern states even spurned free offers of fresh milk and meat from industry donors, preferring to confront the epidemic—as one local newspaper put it—in their own “manly and courageous way.”13
Ultimately, Goldberger realized he was not fighting as much against a human disease as against human nature. “I’m only a bum doctor,” he privately quipped, “What can I do about the economic conditions of the South?”13 Yet for the rest of his life Goldberger remained dedicated to uncovering the elusive “pellagra-preventative (P-P) factor,” which he had confirmed to be in brewer’s yeast by using it to cure dogs of black-tongue disease, the canine manifestation of pellagra. In 1927, when pellagra broke out around the Mississippi River because of flooding, he convinced the Red Cross to distribute brewer’s yeast to the affected regions, and within ten weeks the illness disappeared from those areas. This was Goldberger’s final public triumph, as he died of kidney cancer two years later. Only towards the end of his life was Funk’s model becoming widely accepted. In 1926, the Dutch biochemists B. C. P. Jansen and W. F. Donath isolated the first vitamin, thiamine (B1), in pure form from rice polishings and showed that it could cure beriberi. Then, in 1929, Christiaan Eijkman and Frederick G. Hopkins were jointly conferred the Nobel Prize in Physiology or Medicine for their discovery of anti-neuritic and growth-stimulating vitamins—a few months after Goldberger, who was also nominated, passed away. In the following decade, a total of eight Nobel Prizes were awarded for work on vitamins, and eventually, in 1937, Goldberger’s P-P factor was identified to be niacin (B3) by the American biochemist Conrad Elvehjem. Nutrition was at last recognized as an important aspect of medicine.
Pellagra faded away quietly in the 1940s after niacin was introduced into everyday foods such as bread and cereal. However, over thirty years, it had afflicted three million Americans and claimed more than 100,000 lives, becoming the deadliest dietary disease in U.S. history.1 The tragedy of this epidemic is poignantly reflected by John Steinbeck in The Grapes of Wrath: “There is a crime here that goes beyond denunciation. There is a sorrow here that weeping cannot symbolize. There is a failure here that topples all our success. […] And children dying of pellagra must die because a profit cannot be taken from an orange.”15 On one hand, the story of pellagra is a fascinating exposition of the scientific method, yet on the other hand, it also serves as a stark reminder of how human health and disease are as profoundly shaped by unpredictable societal forces as they are by germs and vitamins.
References
- Bollet, Alfred J. “Politics and pellagra: the epidemic of pellagra in the U.S. in the early twentieth century.” Yale J Biol Med 65 no. 3 (1992): 211-21.
- Babcock, James W. “The diagnosis and treatment of pellagra.” J SC Med Assoc 4 (1908): 563-7.
- Gentilcore, David. “Louis Sambon and the Clash of Pellagra Etiologies in Italy and the United States, 1905-14.” J Hist Med Allied Sci 71 no. 1 (2016): 19-42.
- Funk, Casmir. “The etiology of the deficiency diseases, beri-beri, polyneuritis in birds, epidemic dropsy, scurvy, experimental scurvy in animals, infantile scurvy, ship beri-beri, pellagra.” J State Med 20 (1912): 341-68.
- Lavinder, Claude H., Edward Francis, R. M. Grimm, et al. “Attempts to transmit pellagra to monkeys.” JAMA 63 (1914): 1093-4.
- Siler, Joseph F., Phillip E. Garrison, and Ward J. MacNeal. “A statistical study of the relation of pellagra to use of certain foods and to location of domicile in six selected industrial communities.” Arch Intern Med 14 no. 3 (1914): 293-373.
- Siler, Joseph F., Phillip E. Garrison, and Ward J. MacNeal. “The relation of recurrent attacks of pellagra to race, sex and age of the patient and to treatment of the disease. Arch Intern Med 18 no. 5 (1916): 652-91.
- Mooney, Stephen J., Justin Knox, and Alfredo Morabia. “The Thompson-McFadden Commission and Joseph Goldberger: contrasting 2 historical investigations of pellagra in cotton mill villages in South Carolina.” Am J Epidemiol 180 no. 3 (2014): 235-44.
- Rattini, Kristin B. “The American South’s Deadly Diet.” Discover Magazine. March 2018. http://discovermagazine.com/2018/mar/a-deadly-diet
- Goldberger, Joseph, C. H. Waring, and David G. Willets. “The prevention of pellagra: a test of diet among institutional inmates.” Public Health Rep 30 no. 43 (1915): 3117-31.
- Goldberger, Joseph and G. A. Wheeler. “Experimental pellagra in the human subject brought about by a restricted diet.” Public Health Rep 30 no. 46 (1915): 3336-9.
- Goldberger, Joseph. “The transmissibility of pellagra. Experimental attempts at transmission to the human subject.” Public Health Rep 31 no. 46 (1916): 3159-73.
- Askt, Daniel. “The Forgotten Plague.” American Heritage Magazine. December 2000. https://www.americanheritage.com/content/forgotten-plague
- Goldberger, Joseph, G. A. Wheeler, and Edgar Sydenstricker. “A study of the relation of family income and other economic factors to pellagra incidence in seven cotton-mill villages of South Carolina in 1916.” Public Health Rep 35 no. 12 (1920): 648-713
- Steinbeck, John. The Grapes of Wrath. New York: Penguin Books, 2006. Kindle edition.
PUTZER J. HUNG is a third year medical student at Washington University School of Medicine, where he earned a Ph.D. in immunology in 2018. He is broadly interested in exploring how the scope and practice of medicine evolves with science and society. He grew up on the beautiful Pacific Island of Taiwan and completed his undergraduate education at Brown University.
Highlighted in Frontispiece Volume 11, Issue 1 – Winter 2019

Leave a Reply