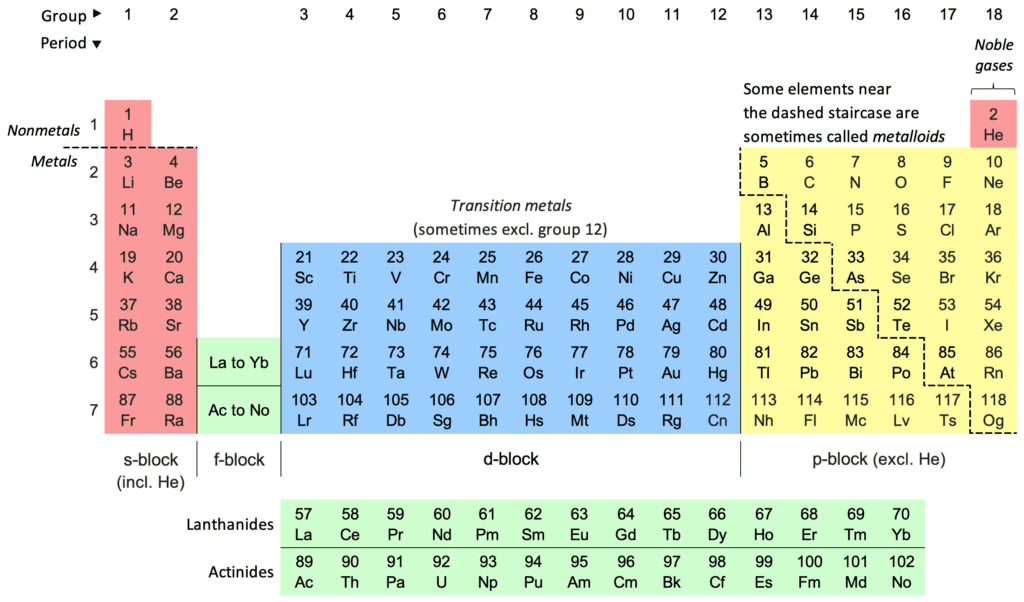
In this system hydrogen is assigned the number one, lithium is three, carbon six, nitrogen seven, oxygen eight, etc. The elements are organized in rows or periods and columns or groups according to their atomic weight. The elements were discovered beginning in the 18th century when the Swedish chemist Carl Wilhelm Scheele discovered oxygen (1772), chlorine, and manganese, followed by Joseph Priestley also independently discovering oxygen (1774), as did Antoine Lavoisier, who also discovered carbon (1783). Nitrogen was officially discovered in 1772 by the Scottish scientist Daniel Rutherford.
In 1869, Mendeleev published his periodic table, which organized elements by their atomic masses and chemical properties. This arrangement allowed him to predict the existence and properties of then-undiscovered elements, such as gallium, germanium, and scandium. Mendeleev’s table was a remarkable achievement that revolutionized chemistry. He was followed in the 20th century by the pioneering work of Ernest Rutherford, Niels Bohr, and Henry Moseley. The current version, often referred to as the “long form” periodic table, consists of 118 elements organized into groups and periods. Elements are grouped based on shared chemical properties and arranged in order of increasing atomic number. This organization allows scientists to make predictions about an element’s behavior and its position in relation to other elements.

Leave a Reply