When the proteins of the human body are broken down to their constituent amino acids, they are converted to ammonia (NH3), which, being toxic, is metabolized in the liver to urea. As the main nitrogenous end product of proteins, urea is found mainly in the blood, but to some extent also in bile, milk, and sweat. It is eventually excreted by the kidneys.
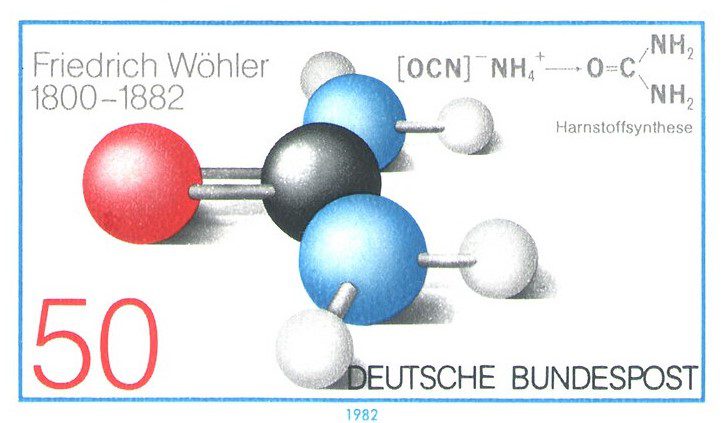
Until the nineteenth century, urea was thought to be an “organic” substance, meaning that it could only be synthesized in living beings—supposedly by the intervention of a “vital” force. But in 1828 the young chemist Friedrich Wöhler added silver cyanate to a solution of ammonium chloride, and, on heating the resulting solution of the unstable compound ammonium cyanate, he found that some white crystals had formed. He recognized that these were urea crystals and realized that, without setting out to do so, he had accidentally synthesized urea in a test tube. Triumphantly, he wrote to his former teacher, the great Swedish chemist Jöns Berzelius: “I can make urea without needing a kidney or even an animal, whether man or dog.” It was a breakthrough and marked the beginning of modern chemistry.
Son of an agronomist and veterinarian, Wöhler was born in 1800 in Eschersheim near Frankfurt on Main in Germany. At first, he had intended to become a physician and at age nineteen enrolled in the faculty of medicine at the University of Marburg. Within one year, however, he switched to chemistry, which he regarded as a more precise and scientific discipline. Graduating in 1823 from the University of Heidelberg, he spent a year in Stockholm under Berzelius and then took up a position in Berlin, where he had a laboratory at his disposal and could conduct his own research. He synthesized not only urea but also other organic compounds such as benzoic and oxalic acids. In 1827, he isolated aluminum in its pure metallic form, followed by yttrium (1828), cesium (1830), and beryllium (1828).
Wöhler also synthesized natural products such as caffeine and quinine, as well as investigating compounds of physiologic importance such as uric acid and cocaine. He was among the first to observe isomerism (compounds having the same chemical formula but different chemical structures). About 1832 he began to collaborate with Justus von Liebig, who taught at Giessen. They studied bitter almond oil (benzaldehyde), a substance in which the same chemical group passes unchanged through a great variety of reactions. They called such a group a radical, the beginning of attempting to systematize organic chemistry.
In 1836 Wöhler became professor of chemistry at the University of Göttingen and remained in that position for forty-six years. An excellent lecturer and teacher, he took a personal interest in his students and contributed to the scientific literature by translating the major works of Berzelius into German. He wrote many books and articles on chemistry and served as one of the editors of Justus Liebig’s Annalen der Chemie, the major chemical journal of its day. A recipient of many honors and awards, he was elected to the Royal Society of London, the French Academy of Sciences, and the Prussian Academy of Sciences. In 1982, West Germany issued a stamp on the 100th anniversary of his death. The stamp depicts the formula for urea and its preparation from ammonium cyanate.

Leave a Reply