Laura Carreras-Planella
Marcella Franquesa
Ricardo Lauzurica
Francesc E. Borràs
Barcelona, Spain
We may think of renal transplantation as routine therapy today, but this procedure has taken centuries to develop and is marked by important events in the history of science. An ancient description of the kidneys is found in the Egyptian Ebers Papyrus, dated to 1550 BC and discovered by the German Egyptologist Georg Ebers (1837-1989). It contained observations made by ancient physicians and included illustrations of human mummies with conditions such as renal cysts or stones. In mummification rites the ancient Egyptians removed all organs from the body except the heart and the kidneys.1 The kidney was believed to be a means of judgment in the afterlife,2, 3 a belief shared by the Jews of Egypt and described in the Old Testament and other ancient writings. The two kidneys were thought to represent good and evil; the right kidney giving a person good advice and the left kidney bad advice. In the afterlife, the kidneys and the heart would be examined to decide the fate of the soul.4 A similar concept is found in traditional Chinese medicine, where the two kidneys represent balance and harmony, hold the yin and yang of the body, determine life and death, and are a reservoir of energy.5
The ancient Greek physician Hippocrates of Kos (460-370 BC) also described diseases and conditions of the kidney and urinary bladder in his Corpus Hippocraticum.6 Aristotle (387-322 BC) proposed an anatomy of the human kidney based on empirical observations of fish and birds. Galen of Pergamum (130-201 AD), one of the most famous Greek physicians and the surgeon of emperors and gladiators, was the first to observe that the major function of the kidneys was to produce urine.7–10 He even introduced the idea that the kidney functioned as a filter. In fact, the word “nephrology” comes from the ancient Greek “νεφρός” (nephros), which is derived from the word “νεφός” (nephos, meaning cloud), and was a metaphorical description of the kidneys producing urine as clouds produce rain.11
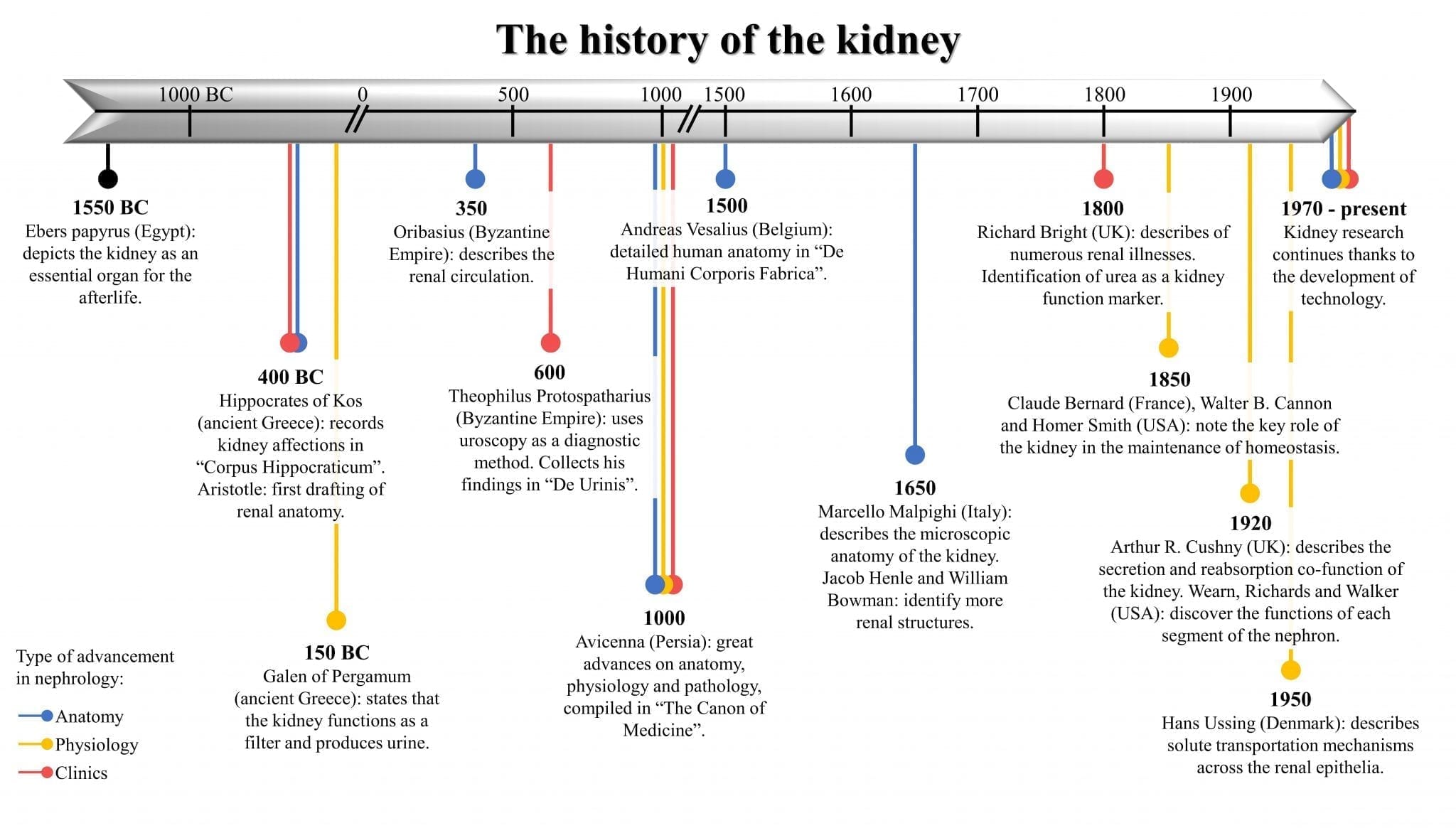 |
| Timeline with the most remarkable hallmarks in the history of the kidney from year 1550 BC to the present. Colors denote the type of advancement in nephrology. Originally created by the authors. |
Some centuries later Oribasius (326-403 AD), physician to the Roman emperor Julian the Apostate, explored the function of the kidneys in Collectiones Medicae. He endeavored to describe the renal circulation, stating that the kidneys absorbed urine from the blood, and also defined the ureters and the urethra.12,13 Although the anatomy had not yet been defined, many disorders and treatments had nevertheless been described. Theophilus Protospatharius (6th-7th century AD) is regarded as the most important uroscopist of the Byzantine Empire; even Emperor Heraklios wished to have him as the physician of the court. Early uroscopists examined the urine for color, turbidity, and sediment to diagnose abnormalities of the kidneys and urinary tract. As this practice evolved, conditions such as diabetes, pregnancy, and liver failure were also diagnosed by uroscopy. Theophilus Protospatharius wrote De Urinis (On Urine), a short book describing findings in urine and renal problems in the elderly. Those descriptions and practices were used by teachers of Western medicine for more than 500 years in famous medical schools such as the Schola Medica Salernitana in Salerno, Italy.14,15
One of the most exceptional physicians in history was Avicenna, also known as Abu Ali al‑Husayn ibn‑Abdullah ibn Sina, who lived in Persia from 980‑1037 AD. He incorporated medical knowledge from his predecessors in ancient Persia and Greece and expanded it with research of his own.16 He even performed clinical trials that were noticeably advanced for that time. His particular focus was the anatomy of the abdomen and the description of the digestive apparatus and associated organs.17,18 Avicenna stated that the liver was the main metabolic organ and that its processed products were filtered and excreted by the kidneys as urine. He hypothesized that since the liver indirectly contributed to the production of urine, analysis of urine could also indicate the health of the liver.19 He compiled his observations in the famous Canon of Medicine.20
During the Renaissance, there were many breakthroughs in anatomy and medicine. Andreas Vesalius (1514-1564), regarded as the father of anatomy, recorded 200 drawings of anatomical structures including the kidney in his masterpiece De Humani Corporis Fabrica.21 He challenged and changed existing theories, such as the concept of sieving through a membrane (membrana cribri malo), which had been incorrect.22,23 In Bologna Marcello Malpighi (1628-1694), who introduced microscopic anatomy, wrote and illustrated documents about the anatomical structure of the kidney. Malpighi identified the glomerulus (also known as the Malpighian corpuscles) and renal tubules.24,25. Other microscopic structures of the kidney were identified later by Jacob Henle (1809-1885)26 and William Bowman (1816-1892)27,28 among others, who made precise drawings and detailed descriptions of their histologic observations of animal and human kidneys.
Around 1820 advances in the clinical field came from Thomas Hodgkin (1798–1866), Thomas Addison (1793-1860), and Richard Bright (1789-1858). They made great contributions to the field of nephrology while working at Guy’s Hospital in London. Richard Bright, who is considered the father of modern nephrology, developed a hospital research unit where he conducted autopsies and described the symptoms of renal diseases.29,30 He was the first to note that albuminuria and edema could be markers of a renal condition that became known as Bright’s disease.31,31
In the field of physiology, Claude Bernard (1813-1878) described how the cells of an organism maintain their function thanks to the constancy of the environment found in the surrounding extracellular fluid space. This state was called Milieu Intérieur (interior milieu), now known as homeostasis and further defined by the American physiologist Walter Bradford Cannon (1871-1945) who also coined the term “fight or flight.”32 This dynamic state of stability is maintained by the kidneys thanks to their capacity for keeping the right amount and composition of substances in the extracellular fluid.33
At the beginning of the twentieth century it was still not clear how secretion and reabsorption could occur at the same time through the epithelium of the kidney tubules, a theory that Arthur Robertson Cushny defended in his monograph The Secretion of Urine published in 1917.34 He also reported the acids found in the urine of humans and other carnivores, whereas herbivores had alkaline urine unless fed a protein diet. Soon after this, Wearn and Richards studied the excretory system of amphibians. Using the recently invented micropuncture technique,35 they sampled fluid filtered through the glomeruli of frogs and reported that its composition was similar to blood except for a lack of proteins.36 Arthur M. Walker and others used oil droplets to block certain segments of the nephron, then injected and collected a known fluid to observe the modifications in that segment and elucidate how the fluid was processed to become urine in the bladder.37 After World War II, Hans Ussing studied the transport of solutes and ions across epithelia,38 and two decades after Burg39,40 developed new techniques for the exploration of the kidney. Devices such as the flame photometer permitted the refined measurement of solutes and led to the understanding of regulation and transport of substances across nephron tubule sections.33
These experiments, descriptions, and inventions have formed the base of our present knowledge about renal pathophysiology and replacement therapy. Dialysis and transplantation are the currently available therapeutic options for end stage renal disease. Of these, transplantation is the best treatment in terms of survival rate, cost-effectiveness, and quality of life. Today over 70,000 kidney transplantations are performed every year worldwide.41–43
The history of kidney transplantation
The history of kidney transplantation as we know it today began in the 1950s, but other key attempts were made earlier in the twentieth century. The first successful organ transplant was performed by Emerich Ullmann from the Vienna Medical School in 1902 when he auto-transplanted a kidney in a dog from its normal location to the vessels of the neck, where it produced some urine.44,45 In the same year, dog-to-dog and dog-to-goat kidney transplants were performed by Ullmann and Alfred von Decastello, who was known for his study of blood groups. In the early 1900s, Alexis Carrel worked closely with the physiologist and surgeon Charles Claude Guthrie, making crucial breakthroughs in vascular surgery such as anastomosis and other techniques for blood vessel preservation. In 1906 Mathieu Jaboulay, with Carrel as assistant surgeon (both of them Nobel laureates), performed the first kidney transplantations from goats and pigs to the arms and thighs of humans. Each kidney worked for one hour only, but these and attempts with other species were performed by pioneers such as Ernst Unger, improving the knowledge of surgical technique.46 The first transplantation from a human cadaver was attempted in the USSR by Yurii Voronoy in 1939, although the organ was rejected because of blood group incompatibility and the patient died after two days.47 Immune mechanisms involved in the grafting of the transplanted organ were poorly understood at the time, as described by Alexis Carrel two years later in a lecture to the International Surgical Society: “The surgical side of the transplantation of organs is now completed, as we are now able to perform transplantations of organs with perfect ease and with excellent results from an anatomical standpoint. But as yet […] transplantations are almost always unsuccessful from the standpoint of the functioning of the organs. All our efforts must now be directed toward the biological methods which will prevent the reaction of the organism against foreign tissue and allow the adapting of homoplastic grafts to their hosts.”48,49
Investigations resumed after World War II with other attempts at human kidney transplantation, especially by two groups in Europe and the United States. In 1946 a human kidney allograft was transplanted to blood vessels in the arm under local anesthesia by a team in Boston.50 The graft only functioned for a short time, but it was long enough to help the patient recover from acute renal failure. This achievement attracted major interest, as did the first transplantation from a live donor performed by Jean Hamburger (who defined the term “nephrology”) in Paris from a mother to her sixteen-year-old son. The transplanted kidney functioned for twenty-two days.51 In 1950, Lawler in Chicago was the first to attempt intra-abdominal kidney transplantation.
In 1954 at Peter Bent Brigham Hospital (later Brigham and Women’s Hospital) in Boston, Joseph Murray performed the first truly successful living donor kidney transplantation. He received the Nobel prize for this achievement in 1990. The transplant was performed from one monozygotic twin to the other, so there was no histo-incompatibility. This was the first time that a transplanted patient, who had been dying from renal failure, survived for years after the transplant.52 The procedure was met with growing success—one kidney recipient even had a successful pregnancy and delivery—and expanded to other hospitals. 53 The first kidney transplantation in Spain was performed in 1965 at the Hospital Clínic de Barcelona by Antoni Caralps, Pedro Pons, Gil-Vernet, and Magriñá, followed by eight additional transplantations at the same hospital that year.
However, even though transplantation surgical techniques had greatly improved, good immunosuppressive regimens were still lacking. The use of the newly available azathioprine, prednisolone, or total body irradiation helped during the initial crucial rejection period between identical twins or siblings.54 In the mid-1960s, great improvements were made in the pre-treatment of patients with hemodialysis to enhance health before surgery; organ transportation between hospitals; identification of HLA antigens, discovered by Jean Dausset; development of tissue-typing and lymphocytotoxicity testing; and an increase in kidney transplants, which provided valuable data for improvement.55–57 Methodologies and management were consolidated in the 1970s, and saw the beginning of transplantations from cadaveric donors.
But the most remarkable breakthrough of this period was the introduction of the calcineurin inhibitors cyclosporine A and tacrolimus. Cyclosporine A was first isolated in 1971 from a soil fungus (Hypocladium inflatum gams) in Norway and studied by Jean-Francois Borel and Hartmann F. Stähelin at Sandoz (now Novartis).58,59 The importance of this drug was reflected in the speed at which it was approved and released to the market in 1983. This small cyclic polypeptide made it possible to reduce the percentage of rejection in the first year after transplantation from 80% to 10%.60 Tacrolimus, somewhat better than cyclosporine A in reducing acute rejection and improving graft survival,61 was isolated from Streptomyces tsukubaensis in the soil of Tsukuba, Japan in 1987. The name tacrolimus derives from “Tsukuba macrolide immunosuppressant,” although it was initially called FK506 because of its target FK506 binding protein (FKBP).62,63,57 Mycophenolic acid, which was first isolated in 1893 from Penicillium glaucum in spoiled corn, was found to possess antibiotic activity but carried many adverse effects.64 A century later, its ester derivate mycophenolate mofetil was synthesized as a safer drug with immunosuppressant action.65,66 Rapamycin, also known as sirolimus and a current first-line immunosuppressant, was first found to be an antifungal metabolite of Streptomyces hygroscopicus. Discovered in Rapa Nui (formerly named Easter Island) in 1964, the name rapamycin comes from the site of its discovery.67–69 It is also abbreviated as mTOR because tor in German means door, and this protein serves as a gateway to cell growth and proliferation.70 Other analogs such as everolimus were synthesized later and are also routinely used in kidney transplantation.71 Although many immunosuppressive drugs are now in use, cyclosporine A and tacrolimus are still key in preventing organ rejection, even fifty years after their discovery.
The modern success of kidney transplantation would not be possible without the previous knowledge acquired by brave, enthusiastic, and brilliant people throughout history who made their findings available for future generations. Discoveries over millennia have made possible a science that today saves thousands of lives.
References
- Salem ME, Eknoyan G. The kidney in ancient Egyptian medicine: where does it stand? Am J Nephrol. 1999;19(2):140-147. doi:10.1159/000013440
- Vargas A, López M, Lillo C, Vargas MJ. El papiro de Edwin Smith y su trascendencia médica y odontológica. Revista médica de Chile. 2012;140(10):1357-1362. doi:10.4067/S0034-98872012001000020
- Taylor, John H. Journey through the Afterlife: Ancient Egyptian Book of the Dead. Harvard Univ Press; 2013.
- Diamandopoulos A, Goudas P. The Role of the Kidney as a Religious, Cultural and Sexual Symbol. American Journal of Nephrology. 2002;22(2-3):107-111. doi:10.1159/000063747
- Maciocia G. The Foundations of Chinese Medicine: A Comprehensive Text. Elsevier Health Sciences UK; 2015.
- Hippocrates, Schiefsky MJ. On Ancient Medicine. Vol 28. Brill; 2005.
- Greydanus D, Kadochi M. Reflections on the Medical History of the Kidney: From Alcmaeon of Croton to Richard Bright – Standing on the Shoulders of Giants. Journal of Integrative Nephrology and Andrology. 2016;3(4):101. doi:10.4103/2394-2916.193496
- Diamandopoulos A, Goudas P. Juxtaposition of Actuarius’ versus Galen’s ideas on renal physiology: the impact of 12 centuries. J Nephrol. 2009;22 Suppl 14:21-32.
- Scarborough J. Galen’s investigations of the kidney. Clio Med. 1976;11(3):171-177.
- Greydanus DE, Merrick J, eds. Medical History: Some Perspectives. Second edition. Nova Biomedical; 2018.
- Michaēlidēs D, ed. Medicine and Healing in the Ancient Mediterranean World. Oxbow Books; 2014.
- Poulakou-Rebelakou E, Marketos SG. Kidney disease in Byzantine medical texts. Am J Nephrol. 1999;19(2):172-176. doi:10.1159/000013446
- Eftychiadis AC. Renal and glomerular circulation according to Oribasius (4th century). Am J Nephrol. 2002;22(2-3):136-138. doi:10.1159/000063751
- Angeletti LR, Cavarra B. Critical and historical approach to Theophilus’ De Urinis. Urine as blood’s percolation made by the kidney and uroscopy in the middle ages. Am J Nephrol. 1994;14(4-6):282-289. doi:10.1159/000168786
- Marketos SG, Eftychiadis AG, Diamandopoulos A. Acute renal failure according to ancient Greek and Byzantine medical writers. J R Soc Med. 1993;86(5):290-293.
- Mazengenya P, Bhikha R. Revisiting Avicenna’s (980–1037 AD) anatomy of the abdominal viscera from the Canon of Medicine. Morphologie. 2018;102(338):225-230. doi:10.1016/j.morpho.2018.05.002
- Gruner OC. A Treatise on the Canon of Medicine of Avicenna. Incorporating a Translation of the First Book. Published online 1984.
- Shah MH. The General Principles of Avicenna’s Canon of Medicine. Vol 1. Naveed Clinic; 1966.
- Madineh SMA. Avicenna’s Canon of Medicine and Modern Urology: part I: bladder and its diseases. Urol J. 2008;5(4):284-293.
- Avicenna 980-1037. A Treatise on the Canon of Medicine of Avicenna, Incorporating a Translation of the First Book. London : Luzac & co., 1930.; 1930. https://search.library.wisc.edu/catalog/999714469602121
- Vesalius A 1514-1564. De Humani Corporis Fabrica : Basel, 1543. Octavo edition. Palo Alto, CA : Octavo; 1998. https://search.library.wisc.edu/catalog/999877146002121
- Cambiaghi M. Andreas Vesalius (1514–1564). Journal of Neurology. 2017;264(8):1828-1830. doi:10.1007/s00415-017-8459-2
- DeBroe ME, Sacré D, Snelders ED, De Weerdt DL. The Flemish Anatomist Andreas Vesalius (1514-1564) and the Kidney. American Journal of Nephrology. 1997;17(3-4):252-260. doi:10.1159/000169110
- Malpighi M. De Viscerum Structura Exercitatio Anatomica. Petrus le Grand; 1669. https://books.google.es/books?id=n-hbAAAAcAAJ
- Motta PM. Marcello Malpighi and the foundation of microscopic anatomy. Prog Clin Biol Res. 1989;295:3-6.
- Kinne-Saffran E, Kinne RK. Jacob Henle: the kidney and beyond. Am J Nephrol. 1994;14(4-6):355-360. doi:10.1159/000168747
- Bowman, William. On the structure and use of the Malpighian bodies of the kidney, with observations on the circulation through that gland. Philosophical Transactions of the Royal Society of London. 1842;132:57-80. doi:10.1098/rstl.1842.0005
- Fine LG. William Bowman’s description of the glomerulus. Am J Nephrol. 1985;5(6):437-440. doi:10.1159/000166979
- Kark RM. A prospect of Richard Bright on the centenary of his death, December 16, 1958. Am J Med. 1958;25(6):819-824. doi:10.1016/0002-9343(58)90055-x
- Young RH. Dr Richard Bright–father of medical renal disease. Arch Pathol Lab Med. 2009;133(9):1365. doi:10.1043/1543-2165-133.9.1365
- Cameron JS. Bright’s Disease Today: The Pathogenesis and Treatment of Glomerulonephritis I. BMJ. 1972;4(5832):87-90. doi:10.1136/bmj.4.5832.87
- Cannon WB. The Wisdom of the Body. New York: WW Norton & Co. Inc; 1932.
- Hoenig MP, Zeidel ML. Homeostasis, the Milieu Intérieur, and the Wisdom of the Nephron. Clinical Journal of the American Society of Nephrology. 2014;9(7):1272-1281. doi:10.2215/CJN.08860813
- Cushny AR. The Secretion of the Urine. Longmans, Green and Company; 1917. https://books.google.es/books?id=a8k0AQAAMAAJ
- Sands JM. Micropuncture: unlocking the secrets of renal function. Am J Physiol Renal Physiol. 2004;287(5):F866-867. doi:10.1152/classicessays.00019.2004
- Wearn JT, Richards AN. Observations on the composition of glomerular urine, with particular reference to the problem of reabsorption in the renal tubules. American Journal of Physiology-Legacy Content. 1924;71(1):209-227.
- Richards AN, Walker AM. Methods of collecting fluid from known regions of the renal tubules of amphibia and of perfusing the lumen of a single tubule. American Journal of Physiology-Legacy Content. 1936;118(1):111-120.
- Ussing HH, Zerahn K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951;23(2-3):110-127. doi:10.1111/j.1748-1716.1951.tb00800.x
- Burg MB, Grantham J, Abramow M, Orloff J, Schafer JA. Preparation and study of fragments of single rabbit nephrons. J Am Soc Nephrol. 1997;8(4):675-683.
- Burg MB, Knepper MA. Single tubule perfusion techniques. Kidney Int. 1986;30(2):166-170. doi:10.1038/ki.1986.168
- Wolfe RA, Ashby VB, Milford EL, et al. Comparison of Mortality in All Patients on Dialysis, Patients on Dialysis Awaiting Transplantation, and Recipients of a First Cadaveric Transplant. New England Journal of Medicine. 1999;341(23):1725-1730. doi:10.1056/NEJM199912023412303
- Mathur AK, Xing J, Dickinson DM, et al. Return on investment for financial assistance for living kidney donors in the United States. Clinical Transplantation. 2018;32(7):e13277. doi:10.1111/ctr.13277
- Tucker EL, Smith AR, Daskin MS, et al. Life and expectations post-kidney transplant: a qualitative analysis of patient responses. BMC Nephrology. 2019;20(1). doi:10.1186/s12882-019-1368-0
- Schlich T. The Origins of Organ Transplantation: Surgery and Laboratory Science, 1880-1930. University of Rochester Press; 2010.
- Carrel A. La technique operatoire des anastomoses vasculaires et la transplantation des viscères. Lyon Med. 1902;98:859.
- Knechtle SJ, Marson LP. Kidney Transplantation: Principles and Practice. 8th ed. Elsevier; 2019.
- Matevossian E, Kern H, Hüser N, et al. Surgeon Yurii Voronoy (1895-1961) – a pioneer in the history of clinical transplantation: in memoriam at the 75th anniversary of the first human kidney transplantation. Transpl Int. 2009;22(12):1132-1139. doi:10.1111/j.1432-2277.2009.00986.x
- Hamilton D. Alexis Carrel and the early days of tissue transplantation. Transplantation Reviews. 1988;2:1-15. doi:10.1016/S0955-470X(88)80003-X
- Carrel A. The transplantation of organs. New York Times. April 14, 1914.
- McGeown MG. Clinical Management of Renal Transplantation. Springer Netherlands; 2013. https://books.google.es/books?id=7WmSBgAAQBAJ
- Michon L, Hamburger J, Oeconomos N. Une tentative de transplantation rénale chez l’homme. Presse Med. 1953;61:1419.
- Carreras-Planella L, Monguió-Tortajada M, Palma È, Borràs FE, Franquesa M. Stem Cells: Immunotherapy in Solid Organ Transplantation. In: Reference Module in Biomedical Sciences. Elsevier; 2018. doi:10.1016/B978-0-12-801238-3.65441-7
- Hatzinger M, Stastny M, Grützmacher P, Sohn M. [The history of kidney transplantation]. Urologe A. 2016;55(10):1353-1359. doi:10.1007/s00120-016-0205-3
- Marcén R. Immunosuppressive Drugs in Kidney Transplantation: Impact on Patient Survival, and Incidence of Cardiovascular Disease, Malignancy and Infection. Drugs. 2009;69(16):2227-2243. doi:10.2165/11319260-000000000-00000
- Starzl TE, Marchioro TL, Waddell WR. The reversal of rejection in human renal homografts with subsequent development of homograft tolerance. Surg Gynecol Obstet. 1963;117:385.
- Collins GM, Bravo-Shugarman M, Terasaki PI. Kidney preservation for transportation. Initial perfusion and 30 hours’ ice storage. Lancet. 1969;2(7632):1219-1222. doi:10.1016/s0140-6736(69)90753-3
- Starzl TE, Todo S, Fung J, Demetris AJ, Venkataramman R, Jain A. FK 506 for liver, kidney, and pancreas transplantation. Lancet. 1989;2(8670):1000-1004. doi:10.1016/s0140-6736(89)91014-3
- Heusler K, Pletscher A. The controversial early history of cyclosporin. Swiss Med Wkly. 2001;131(21-22):299-302. doi:2001/21/smw-09702
- Pritchard DI. Sourcing a chemical succession for cyclosporin from parasites and human pathogens. Drug Discovery Today. 2005;10(10):688-691. doi:10.1016/S1359-6446(05)03395-7
- Calne R. Cyclosporine as a milestone in immunosuppression. Transplant Proc. 2004;36(2 Suppl):13S-15S. doi:10.1016/j.transproceed.2004.01.042
- Ekberg H, Grinyó J, Nashan B, et al. Cyclosporine sparing with mycophenolate mofetil, daclizumab and corticosteroids in renal allograft recipients: the CAESAR Study. Am J Transplant. 2007;7(3):560-570. doi:10.1111/j.1600-6143.2006.01645.x
- Kino T, Hatanaka H, Hashimoto M, et al. FK-506, a novel immunosuppressant isolated from a Streptomyces. I. Fermentation, isolation, and physico-chemical andbiological characteristics. J Antibiot. 1987;40(9):1249-1255. doi:10.7164/antibiotics.40.1249
- Hatanaka H, Iwami M, Kino T, Goto T, Okuhara M. FR-900520 and FR-900523, novel immunosuppressants isolated from a Streptomyces. I. Taxonomy of the producing strain. J Antibiot. 1988;41(11):1586-1591. doi:10.7164/antibiotics.41.1586
- Alsberg, C L., Black, O. F. Contributions to the study of maize deteriotation. U S Dept Agr Plant Ind Bull. 1913;270:5-48.
- Bullingham RES, Nicholls AJ, Kamm BR. Clinical Pharmacokinetics of Mycophenolate Mofetil: Clinical Pharmacokinetics. 1998;34(6):429-455. doi:10.2165/00003088-199834060-00002
- van Gelder T, Hesselink DA. Mycophenolate revisited. Transpl Int. 2015;28(5):508-515. doi:10.1111/tri.12554
- Chung J, Kuo CJ, Crabtree GR, Blenis J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell. 1992;69(7):1227-1236. doi:10.1016/0092-8674(92)90643-Q
- Kuo CJ, Chung J, Fiorentino DF, Flanagan WM, Blenis J, Crabtree GR. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature. 1992;358(6381):70-73. doi:10.1038/358070a0
- Price D, Grove, Calvo V, Avruch J, Bierer B. Rapamycin-induced inhibition of the 70-kilodalton S6 protein kinase. Science. 1992;257(5072):973-977. doi:10.1126/science.1380182
- Heitman J. On the Discovery of TOR As the Target of Rapamycin. PLOS Pathogens. 2015;11(11):e1005245. doi:10.1371/journal.ppat.1005245
- Ventura-Aguiar P, Campistol JM, Diekmann F. Safety of mTOR inhibitors in adult solid organ transplantation. Expert Opinion on Drug Safety. 2016;15(3):303-319. doi:10.1517/14740338.2016.1132698
LAURA CARRERAS-PLANELLA, BSc, is a PhD student in Advanced Immunology at the Autonomous University of Barcelona, where she studied Veterinary Medicine. She is now working on proteomics for biomarker discovery in renal transplantation under the supervision of Dr. Francesc E. Borràs. She is enthusiastic about medical and scientific discoveries and hopes to make basic research more translatable to patients.
MARCELLA FRANQUESA, MSc, PhD, obtained her PhD in Biomedicine in 2010 in the Experimental Nephrology Department at Hospital de Bellvitge–IDIBELL under the direction of Dr. Josep M Grinyó. After post-doctoral experiences in Prof. Bonventre’s laboratory in Brigham and Women’s Hospital—Harvard Medical School in Boston, and at the Transplantation Laboratory of the Erasmus Medical Center in Rotterdam, Netherlands, under the supervision of Martin Hoogduijn and Carla Baan, in 2015 she joined the REMAR-IVECAT group at IGTP (Barcelona) as principal researcher.
RICARDO LAUZURICA, MD, PhD, is clinical Chief of the Nephrology service and Director of the Renal Transplantation Unit of the Germans Trias i Pujol University Hospital. He completed the specialty in Nephrology in Clínica Puerta de Hierro (Madrid) and completed a fellowship in the Kidney Transplant Unit in Hospital Necker (Paris). In the last 10 years, he has been principal investigator in more than 30 clinical trials (phase II, III, and IV) in kidney transplantation mostly focused on immunosuppressive therapies. He has participated in more than 100 published scientific articles in indexed journals. Member of several relevant nephrology related societies.
FRANCESC E. BORRAS, MSc, PhD, obtained his PhD in Immunology in 1998, and joined the Dept. of H&I at the NBS in London (UK) led by Dr. Cristina Navarrete. Given his interest in transplantation, in 2002 he joined the Immunology lab at the Germans Trias i Pujol Research Institute (IGTP). Since 2014, he coordinates the REMAR-IVECAT Group at IGTP, studying and addressing the problems of kidney diseases. A former member of the board of the Catalan Society for Immunology (2008-12) and former Editor of Inmunología (2010-13), he is currently Assistant professor of Immunology at the University of Barcelona and Vice-president of GEIVEX (Spanish Society for Extracellular Vesicles).
Summer 2020 | Sections | Nephrology & Hypertension

Leave a Reply