Kevin R. Loughlin
Boston, Massachusetts
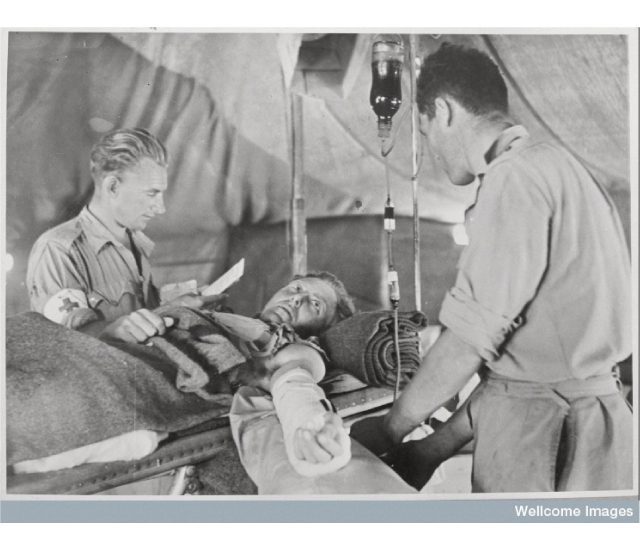
The rudimentary lights provided only dim illumination of the operative field. The three British army surgeons worked feverishly to save the life of the young soldier, Corporal Smith, who had a significant liver injury. He had already lost a liter of blood during transport from the front. As the surgeons continued their work, the anesthesiologist hung a third pint of blood. The Battle of Cambria, one of the bloodiest battles of World War I, raged on in the hillsides of northern France, a few weeks before Christmas in 1917.
The young corporal ultimately survived, in large measure because of the blood transfusions he received, which had only recently become available owing to medical research. If the same circumstance had occurred only twenty years earlier without the availability of blood transfusions, it is unlikely that the soldier would have survived.
William Harvey and early experience with blood transfusions
Since 1628 when William Harvey discovered the circulation of blood, there had been hope that blood transfusion would be possible. Harvey expressed the awe in which physicians of the time regarded blood when he said, “We conclude that blood lives of itself and that it depends in no ways upon any parts of the body. Blood is the cause not only of life in general, but also of longer or shorter life, of sleep and waking, of genius, aptitude and strength. It is the first to live and the first to die.”1
After Harvey’s discovery, transfusion attempts began. In 1665 Richard Lower kept dogs alive by transfusing blood from other dogs.2 In 1667 French physician Jean Denys transfused nine ounces of blood from the carotid artery of a lamb into the vein of a young man. He continued the practice until the third patient so treated, died.3 Denys was sued by the wife of the deceased patient, who presumably died from a hemolytic reaction, but was exonerated. However, the French Parliament, the Royal Society, and the Catholic Church subsequently issued a general prohibition against transfusions.4
It would not be until 1818 when transfusions were seriously considered again. A British obstetrician, James Blundell, performed a human blood transfusion in the setting of a postpartum hemorrhage.5 However, the debate over transfusions continued over the remainder of the nineteenth century. In 1849 C.H.F. Routh reviewed all the published transfusions to date and remarked in the Medical Times that of the 48 recorded cases, 18 had a fatal outcome and concluded that the mortality rate was unacceptably high.5 The next major advance in transfusion therapy would wait until the turn of the century.
Landsteiner, Hektoen, and the foundations of modern transfusion therapy
Karl Landsteiner was an Austrian physician and immunologist. While working at the University of Vienna, he became interested in blood serum work, specifically the factors that led to hemagglutination of red blood cells. This resulted in two landmark publications in 1900 and 1901 that described the evidence of blood groups that he named A, B, and C.6,7 These would later be modified to A, B, and O. Two years later, two of his colleagues, Alfred Von Decastelo and Adriano Sturli, would add a fourth blood type, AB.8,9 Landsteiner would be awarded the Nobel Prize in 1930 for his elucidation of the blood groups.
In 1907 Ludvig Hektoen, professor and chief of pathology at the University of Chicago and the Cook County Hospital made the important observation that “the common occurrence of isoagglutinins in human serum suggests that under certain special conditions transfusions might prove dangerous by leading to erythrocyte agglutination within the vessels of the subject transfused.”10 This critical observation led to an appreciation that cross-matching between donor and recipient would increase the safety of blood transfusions.11 Soon thereafter, in 1912, Doctor Roger Lee demonstrated that O blood could be given to a person of any blood type (universal donor) and that a person with AB blood could receive blood from any blood group (universal recipient).
The most important medical advancement of the war
World War I introduced the weapons of modern warfare and with it more severe trauma. Advances in transfusion science paralleled the increased need for and use of transfusions on the battlefield. Two individuals, not related, Lawrence Bruce Robertson and Oswald Hope Robertson, deserve special mention. Stansbury and Hess referred to the acceptance of the life-saving potential of blood transfusions in the resuscitation of combat casualties as “the most important medical advance of the war.”12
Lawrence Bruce Robertson was a Canadian who received his medical degree at the University of Toronto. He did postgraduate training in Boston and New York and learned the syringe method of transfusion as a means of mitigating clotting from Edward Lindemann at Bellevue. L.B. Robertson enlisted in the Canadian army in 1914 and on arriving in Europe championed the use of whole blood as the treatment of choice for battlefield casualties.13 This was a major medical advance and his experience was published in the British Medical Journal with the suggestion “for its more frequent employment in war surgery.”14 It should be noted that Robertson’s practice was contrary to the accepted medical dogma of the day. Less than a decade earlier, one of the surgical giants of the era, George Crile, had opined in the same British Medical Journal: “Excellent results were certainly obtained in some cases of shock, but in the treatment of this condition, and indeed, of all others in which intravascular infusion of some kind is clearly indicated, surgeons, we imagine, will find no good reasons given here for abandoning the safe and simple method of saline injection.”15
Oswald Hope Robertson likewise made significant contributions to the management of battlefield trauma. O.H. Robertson was born in London and as a young child moved to California with his family. He studied at the University of California, Harvard Medical School, and the Rockefeller Institute. At Harvard he worked with Roger Lee on blood transfusion research.16 At Rockefeller he worked with Peyton Rous and was exposed to blood typing and red blood cell storage techniques. However, he cut short his studies to join the medical teams in France. While in France, he developed a glass transfusion bottle using the Rous-Turner solution of citrate and glucose to serve as a preservative. An entry from November 30, 1917 in O.H. Robertson’s diary captures the stress of managing the trauma from the modern weapons of warfare with the less than modern, somewhat rudimentary, instruments of medicine. Robertson wrote, “The beds were filled and we began putting stretchers on the floor. Hemorrhage, hemorrhage, hemorrhage- blood everywhere-clothes soaked in the blood, pools of blood in the stretchers, streams of blood dripping from the stretchers to the floor.”12 Robertson’s experience and subsequent publications helped transform the management of wartime trauma.17,18 The rapidity of change in medical opinion is demonstrated by contrasting the comments of Harvey Cushing with those of Crile just slightly more than a decade before. Cushing commented, “Good hospitals are performing 50 transfusions a day.”19
Post World War I advances
As blood transfusions became more widespread in medical practice, the concept of establishing blood banks became attractive. In the 1930s Bernard Fantus at Cook County Hospital20 and Carl W. Walter at Peter Bent Brigham Hospital started blood banks. In Boston, Walter’s efforts were viewed with such skepticism and disdain that his facility was relegated to a basement room at Harvard because some trustees thought the storage and use of blood was “immoral and unethical.”21 Fifteen years later he invented the plastic blood bag, which greatly facilitated transfusion therapy.21
In 1939 Levine and Stetson reported a case of isosensitization in a woman who had delivered a stillbirth at Bellevue Hospital.22 They postulated that there had been an isosensitization in the woman caused by “products” of the fetus. A year later the discovery by Landsteiner, still productive in his later years, and Alexander Weiner of the Rh factor, provided the pathophysiologic basis for erythroblastosis and likely explained the Levine case from the prior year.23 The elaboration and description of the Rh factor contributed to the further sophistication of transfusions and blood banking.
In 1940 Edwin Cohn developed ethanol fractionation, the process of breaking down plasma into component products. Albumin, gamma globulin, and fibrinogen were isolated to become available for clinical use.
World War II and the modern era
In 1944 dried plasma became available for the treatment of combat injuries. Component transfusion therapy became more widely used as the war progressed. The Red Cross concluded its World War II blood program in 1945 after 13 million pints had been collected.11
In 1961 platelet concentrates became recognized for reducing mortality from hemorrhage in cancer patients. In 1964 plasmapheresis was introduced as a means of collecting plasma for fractionation. In 1971 Hepatitis B surface antigen (HbsAg) testing of donated blood began and in 1992 testing of donor blood for HIV-1 and HIV-2 antibodies commenced.
The current landscape
Fast forward to the Second Battle of Fallujah. It is December of 2004, almost eighty-seven years to the day after the Battle of Cambria, and another battle rages in northern Iraq. The similarities as well as the differences are striking. Young soldiers are still suffering life-threatening injuries. Another Corporal Smith is brought to the operating room with a serious liver injury. An anti-shock garment has been placed on him at the battle front and the transport is significantly faster.
He is triaged quickly and appropriate specialists are brought to the OR. Modern telemetry monitors his moment-to-moment status. Component transfusions of packed red blood cells, whole blood, platelets, and fresh frozen plasma are administered as needed. After the abdomen is opened, a cell saver suction tubing is placed in the operative field so that his own blood can be collected and autotransfused back to him.
Gawande’s data
Atul Gawande has chronicled the impressive success of military medicine in reducing wartime fatalities. He states that although the firepower has increased, lethality has decreased.24 He reports that in World War II, 30% of Americans injured in combat died. That mortality rate decreased to 24% in the Vietnam War and in the war in Iraq and Afghanistan it dropped still further to about 10%. Although this decrease in mortality is due to several factors, the scientific advances that occurred in the understanding of the many aspects of blood transfusion serve as the underpinning for much of this success.
References
- William Harvey, Science Quotes, accessed 12/31/19
- Roth GA. The blood donation evolution. https://circulatingnlm.nih. gov/2013/08/09/the blood-donor-evolution/ accessed 12/31/2019
- Schwarz HP and Dorner F. Karl Landsteiner and his major contributions to hematology. Brit. J. Haematology 2003; 121(4):1365
- Blakemore C and Jennett S (eds) 2001, The Oxford Companion to the Body. Oxford University Press, New York
- Learoyd P. A Short History of Blood Transfusion, National Blood Service (January 2006) accessed 12/30/19
- Landsteiner K Zur Kenntnis der antifermentativen, lytischen und agglutinierenden Wirkungen des Blutserums und der Lymph. Centralblatt fur Bacteriologie 1900; 27: 357-352
- Landsteiner K Ueber Agglutinationserscheinungen normalen menschlichen Blutes. Wiener Klinische Wochenschrift 1901; 46: 1132-1134
- Von Decastelo A and Sturli A. Uber die Isoagglutinine in Serum gesunder und kranker Menschen. Muenchener Medizinische Wochenschrift 1902; 49:1090-1095
- Stankus K. A brief history of blood transfusion through the years, The Stanford Blood Center. https://stanfordbloodcenter.org/a-brief-history-of-blood-transfusion-through-the-years/ accessed 10/15/19
- Cannon PR. Ludvig Hektoen 1863-1951: A Biographical Memoir. National Academy of Sciences, Washington, DC, 1954
- History of Blood Transfusion. American Red Cross. Accessed 12/23/19
- Stansbury LG and Hess JR. Blood transfusion in World War I: The Roles of Lawrence Bruce Robertson and Owen Hope Robertson in the “Most Important Medical Advance of the War” Trans. Med. Rev. 2009;23(3): 232-236
- Maxwell A. Dr. Lawrence Bruce Robertson and blood transfusion in the trenches of World War I. Canadian Blood Services. https://blood.ca/en/research/our-research-stories/research-education-discovery/dr.lawrence.b.robertson. Accessed 12/31/19
- Robertson LB. The transfusion of whole blood: A suggestion for its more frequent employment in war surgery. Br. J. Med. 1916;2:38-40
- Crile GW. The transfusion of blood. Br. J. Med. 1907;2:1007
- Stansbury LG and Hess JR. Putting the pieces together: Roger I. Lee and modern transfusion medicine. Trans. Med. Rev. 2005; 19: 81-84
- Robertson OH. A method of citrated blood transfusion. Br. J. Med. 1918; 1:477-479
- Robertson OH. Transfusion of preserved red cells. Br. J. Med. 1918; 1:691-695
- Cushing H. From a Surgeon’s Journal. Boston Little Brown and Co. 1936
- Blood transfusion. Wikipedia. https://en.wikipedia.org/wiki/Blood_transfusion. Accessed 10/15/2019
- Obituary- Dr. Carl W. Walter; Inventor of Blood Bag. Los Angeles Times , May 10,1992
- Levine P and Stetson RE. An unusual case of intragroup agglutination. Journal of the American Association. 1939; 113:126
- Landsteiner K and Wiener AS. An agglutinable factor of human blood recognizable by immune sera for rhesus blood. Proceedings of the Society for Experimental Biology and Medicine 1940; 43:223
- Gawande A. Casualties of War- Military Care for the Wounded from Iraq and Afghanistan. N. Engl. J. Med. 2004; 351: 2471-2475
KEVIN R. LOUGHLIN, MD, MBA, is a retired urologic surgeon who practiced at Brigham and Women’s Hospital for thirty-five years. He is a professor emeritus at Harvard Medical School and a trustee emeritus of the American Board of Urology.
Submitted for the 2019–2020 Blood Writing Contest & Highlighted in Frontispiece Volume 12, Issue 2 – Spring 2020

Leave a Reply