Ashok Singh
Chicago, Illinois, United States
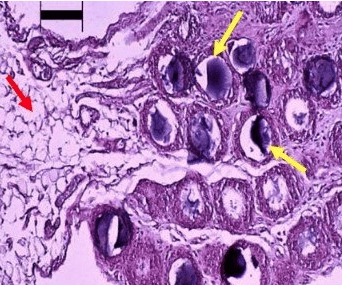 |
| Histology of activated omentum 3 days after placing a 5 cc slurry of inert polydextran particles of approx. 100 micron diameter (1 million particles) in the abdominal cavity of rats. Note the dramatic change in the size and quality of the omentum. While the native omentum is fatty in nature (as seen in left one-third of the picture; red arrow), the activated omentum in contrast appears to be a compact tissue surrounding each inert particle (as see in right two-thirds of the picture; yellow arrows point to polydextran particles). Black bar on the above left of the picture represents a length of 100 microns. Photo by the author, source. |
The omentum is a curtain-like tissue that hangs from the bottom edge of the stomach and covers the abdominal organs below. It is a lattice of adipose (fat) cells peppered with islands of compact tissue known as milky spots, which are clusters of macrophages, lymphocytes, and hematopoietic cells. The omentum extends and contacts injured sites in the abdomen, fuses with them, and initiates vascularization, debridement, hemostasis, and repair. Such salutary properties have long been recognized by surgeons, as the omentum can stop the bleeding from minor accidental cuts during abdominal surgery.
The omentum is often deliberately sutured to injured organs in the abdomen for the same reason. For example, it may be used as a tissue bandage around a surgical gastrointestinal anastomosis to reinforce the joint and prevent leakage. It is also routinely used to plug perforations caused by gastric or duodenal ulcers, forming new blood vessels that will serve as scaffolding for a fibrous closure. In a liver resection, covering the remaining stump with omentum prevents bleeding and allows severed blood vessels to heal. Crush injuries of the spleen may be treated by draping the omentum around the damaged area to restore function; it also helps in reconstruction of the abdominal wall after major surgery.
Tissue repair by the omentum to organs of the body outside the abdomen is also possible by tailoring the omentum to form a long pedicle, keeping intact its major blood vessels, and then suturing the pedicle to the injured organ. The procedure sometimes called omental transposition has been used to improve reconstruction of arterial grafts using synthetic materials in remote areas of the body. Omental pedicle wrapping has been used in chest wall reconstruction following radical thoracic surgery; the omentum helps in the integration of synthetic scaffolding mesh with host tissue and prevents infection at the site. Omental transposition in combination with coronary bypass improves functional capacity after ischemic heart failure. Exceptional anecdotal examples include tunneling the long omental pedicle subcutaneously to the brain to restore memory in Alzheimer’s patients; to recover motor and sensory function in stroke patients; to stimulate nerve growth and reverse paralysis in spinal cord injury; and to regain urinary control in neurogenic bladder. Many other applications have been reported in orthopedic, gynecological, and urogenital surgeries.
The omentum has been widely used in surgical practice for years, but its remarkable properties were never fully explained until a research group at Cook County Hospital in Chicago undertook a series of experiments. They simulated an injury in the abdominal cavities of rats using a slurry of inert particles (~100 microns in size) to challenge the omentum. The omentum became “activated” as it recognized the foreign body and dramatically extended itself to surround each particle within two to three days. The activated omental tissue was not made up of its original fat cells, but instead was a highly compact and vascular tissue—a giant milky spot. Biochemical and histological analyses showed that the activated omentum contained all the hallmarks of a regenerated tissue. It contained cells that behaved like stem cells, secreting copious amounts of paracrine growth factors (such as vascular endothelial growth factor and connective tissue growth factor) when cultured in Petri dishes.1 These extraordinary findings spurred investigation into new applications. In further work, the Chicago group showed that dead β-cells in rats with Type 1 diabetes could be revived to produce insulin after bits of the pancreas were placed in activated omentum.2 Dr. Christof Westenfelder and his group in Salt Lake City, Utah3 found that cultured insulin-producing cells combined with mesenchymal stem cells (isolated from fat tissue) that were grafted to the omentum of diabetic mice spontaneously formed functional neo-islets and achieved long-term glycemic control without immunosuppression. This novel technology has significant translational relevance for Type 1 diabetes, as it effectively overcomes the obstacles of pancreas organ donor scarcity and the need for immunosuppression.
Rutherford Morrison, a British surgeon, observed the mobility of the omentum to sites of injury and in his 1910 article “Introduction to Surgery” called it “the policeman of the abdomen.” With modern knowledge of the functions of the omentum, this may have been a great understatement.
References
- Litbarg, N, Gudehithlu KP, Sethupathi P, Arruda JAL, Dunea, G, Singh AK: Activated omentum becomes rich in factors that promote healing and regeneration. Cell Tiss Res 328: 487-497, 2007.
- Singh AK, Gudehithlu KP, Litbarg, N, Sethupathi P, Arruda JAL, Dunea, G: Transplanting fragments of diabetic pancreas into activated omentum gives rise to new insulin producing cells. Biochem Biophys Res Comm 355: 258-262, 2007.
- Westenfelder C, Gooch A, Hu Z, Ahlstrom J, Zhang P: Durable Control of Autoimmune Diabetes in Mice Achieved by Intraperitoneal Transplantation of “Neo‐Islets,” Three‐Dimensional Aggregates of Allogeneic Islet and “Mesenchymal Stem Cells”. Stem Cells Translational Medicine 6: 1631-1643, 2017.
ASHOK SINGH, PhD, is a medical scientist who obtained a doctorate in experimental biochemistry and immunochemistry. After several academic positions at US medical centers, he became the Principal Scientist at the Cook County Hospital in Chicago IL (USA). He has published more than 90 peer-reviewed research papers in the field of nephrology, including in membranous nephropathy, diabetic nephropathy, and proteinuria. In his later career, his contributions were in angiogenesis, wound healing, and stem cells. He founded and currently runs a small stem cell company, Vivastem Laboratories in Chicago, catering to the health of companion animals (dogs, cats, horses) using a unique technology he patented and developed.
Highlighted in Frontispiece Volume 13, Issue 3– Summer 2021
Winter 2021 | Sections | Science

Leave a Reply