Ashok Singh
Chicago, IL, United States
 |
 |
|
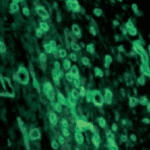
Tissue Richly Endowed with Blood Vessels |
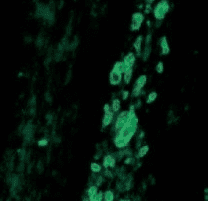
Loss of blood vessels after the same tissue was treated with Anti-VEGF antibody |
Various cells in the human body, such as lymphocytes, monocytes, and all tissue cells release small proteins that, unlike hormones, which act at distant sites, have powerful effects on only neighboring cells. These proteins go under a variety of names such as paracrine factors, growth factors, or, more generally, cytokines and are released under conditions of stress, bleeding, inflammation, infection, injury, and wound healing. At least fifty such factors have been recognized and well-studied.1 Examples of such factors are vascular endothelial growth factor (VEGF), fibroblast growth factor (FGF), transforming growth factors (TGF), interleukins, interferon, and clotting factors.
One of the most important paracrine factors is VEGF, which in the fetal as well as adult state stimulates the growth of blood vessels. This activity is known as vasculogenesis, angiogenesis, or neovascularization. During bleeding and wound healing, VEGF is produced by monocytes that migrate to the injured site in order to build a network of blood vessels that bring about repair and regeneration of the injured tissue. Interestingly, tumor cells were also found to be capable of producing large amounts of VEGF. VEGF produced by tumor cells promotes the growth of the solid tumor by stimulating the extension and branching of blood vessels that is crucial for metabolically supporting the expanding tumor mass.
The discovery of VEGF is a story not uncommon in the scientific world, that is, it was recognized at different times as a different molecule, only to be finally unraveled as one molecule. Research in this field was spearheaded by Dr. Judah Folkman and his collaborators, who in 1970 isolated a factor from extracts of solid tumors that could induce proliferation of new blood vessels, and accordingly named it tumor angiogenesis factor. Later, in 1983, Senger et al., following the lead that many abdominal tumors cause an accumulation of ascites fluid in the abdomen, started to investigate the ascites fluid and discovered in it a similar factor they called vascular permeability factor. In 1989 Ferrara and Henzel described an angiogenic factor in bovine pituitary follicular cells (similar in activity to that found by Folkman earlier), which they purified, cloned, and named vascular endothelial growth factor (VEGF). When the above studies were compared, it turned out that the three groups were all independently describing the same factor, which by consensus was officially named VEGF. Later on, other accessory angiogenic factors, FGF, angiopoietin-1 and 2 etc., were found that worked synergistically with VEGF to form fully functional blood vessels. The VEGF gene in the DNA of mammals exists as a split gene spread linearly in eight fragments (called exons) separated by non-coding DNA sequences called introns. Different combinations of the exons during transcription (alternate splicing) results in five isoforms of VEGF (VEGF-A, VEGF-B, C, D, and placenta growth factor (PGF)). Each of these different isoforms is critically involved in forming and maintaining tissue-specific blood and lymphatic vessels. The VEGF family of factors act by interacting with receptors on responsive cells called neuropilins.
Recognition of a specific factor responsible for the growth of blood vessels was a pivotal finding because it offered a novel approach to target tumors by way of antagonizing VEGF. Soon it was hypothesized that since tumors needed blood vessels in order to grow, cutting off their blood supply would impede their further growth and induce a remission of the tumor. Humanized monoclonal antibodies directed against VEGF were developed by Ferrara’s group for this purpose. In 2004, the first such antibody, bevacizumab (Avastin), was approved by the FDA as a treatment for colon cancer. A similar drug, Lucentis, was later also approved for use. While both Avastin and Lucentis have been successful as anti-cancer agents, their widespread off-label use for eye diseases is no less a fascinating and triumphant story (summarized below from ref 2).
It is well known that abnormal growth of blood vessels of the retina is a leading cause of vision loss and blindness. In 1948 Michaelson hypothesized that there was a diffusible factor (“Factor X”) that causes abnormal vascular proliferation and leakage.3 A frantic search began to identify Factor X. Intrigued by the discovery of VEGF, Adamis and his team in the 1990s started to wonder whether VEGF could be that elusive Factor X. They grew retinal pigment epithelium cells in culture and showed that they secreted VEGF, the first evidence that these cells produce this factor. They went on to collect ocular fluid from ischemic retinopathy induced in non-human primates. When they examined the fluid for VEGF, they found that it was absent before neovascularization but rose very quickly to high levels when blood vessels proliferated, and decreased as blood vessels regressed. Soon after, two groups, one consisting of D’Amico, Folkman, Adamis, and Miller and the other of Aiello, King, and Ferrara collected ocular fluid from human patients with proliferative diabetic retinopathy and found that VEGF levels correlated with neovascularization, whereas the VEGF levels were low in eyes with quiescent disease or in normal control eyes. The group of Adamis, Miller, and Folkman further showed that intra-vitreal injection of VEGF recapitulated the proliferative eye disease, including all its hallmarks: new blood vessels in the iris, neovascular glaucoma, retinal ischemia, and microangiopathy. Further, using the ischemic retinopathy and iris neovascularization model in animals, they tested an anti-VEGF antibody (essentially the precursor to Avastin), and found that intra-vitreal injection of the antibody prevented iris neovascularization, whereas a control antibody had no effect. Similar results were obtained by Aiello’s group in a mouse model of retinopathy in which they used a different approach of blocking VEGF using a soluble VEGF receptor. These results established that VEGF inhibition in the eye resulted in decreased neovascularization. Later these researchers collaborated with pharmaceutical companies Genentech and Eyetech Pharmaceuticals to develop the VEGF antibodies Avantis and Lucentis for age-related macular degeneration (AMD) and other proliferative retinal diseases. Today more than a million eye patients are treated every year with Avantis and Lucentis. This treatment has resulted in >90% of patients avoiding moderate vision loss and 30% of treated patients attaining a vision of 20/40 or better—a stunning achievement.
Image Credit
- The power of anti-VEGF antibody in cutting off blood vessels in a tissue illustrated by treating a foreign-body induced granulation tissue induced in the subcutaneous space with anti-VEGF antibody. Images by the author. Source: Singh et al: Impaired integration of endothelial progenitor cells in capillaries of diabetic wounds is reversible with vascular endothelial growth factor infusion. Translational Research 2007; 149.
References
- Werner S, Grose R: Regulation of Wound Healing by Growth Factors and Cytokines. Physiol Rev 83: 835-870, 2003.
- Miller JW: VEGF: From Discovery to Therapy: The Champalimaud Award Lecture. Trans Vis Sci Tech 5: 1-13, 2016.
- Michaelson IC. The mode of development of the vascular system of the retina, with some observations on its significance for certain retinal diseases. Trans Ophthalmol Soc U K. 68:137–180, 1948.
ASHOK SINGH, PhD, is a medical scientist who obtained a doctorate in experimental biochemistry and immunochemistry. After several academic positions at US medical centers, he became the Principal Scientist at the Cook County Hospital in Chicago IL (USA). He has published more than 90 peer-reviewed research papers in the field of nephrology, including in membranous nephropathy, diabetic nephropathy, and proteinuria. In his later career, his contributions were in angiogenesis, wound healing, and stem cells. He founded and currently runs a small stem cell company, Vivastem Laboratories in Chicago, catering to the health of companion animals (dogs, cats, horses) using a unique technology he patented and developed.

Leave a Reply